Philips, today announced that Taipei Veterans General Hospital (TPVGH) will utilize the Philips IntelliSite Pathology Solution to establish Taiwan’s first fully digital pathology department. By allowing tissue samples to be remotely viewed within a virtual pathology network across hospital locations, the Philips IntelliSite Pathology Solution will help TPVGH enhance patient safety and improving the quality of diagnoses, thereby leading to better patient outcomes.Impact of Cancer in TaiwanCancer
Read More
Philips’ IntelliSite Solution
Jackson Health Expands 11-Year Partnership with Philips with End-to-End Managed Service for Healthcare IT
Philips, today announced that Jackson Health System is expanding on the world’s first, 11-year long-term strategic partnership for Enterprise Monitoring as a Service (EMaaS) by implementing IntelliSpace Enterprise Edition, including Philips PerformanceBridge. Philips IntelliSpace Enterprise Edition is a scalable, interoperable and secure end-to-end managed service for healthcare IT. By expanding the partnership, the two are taking their long-term collaboration forward to drive meaningful
Read More
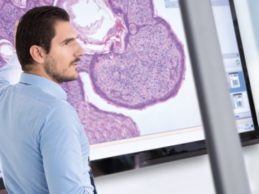
Philips Partners With Mass General to Advance Digital Pathology Adoption
Philips announced it’s collaborating with Massachusetts General Hospital (MGH) and Brigham and Women’s Hospital (BWH) to enable research, support clinical diagnosis and help advance digital pathology adoption across the U.S. through the implementation of the Philips IntelliSite Pathology Solution.This collaboration builds on digital pathology’s growing momentum in recent years, due to its ability to enable highly automated workflows that allow pathologists to compile clinically actionable
Read More
Philips Awarded FDA Clearance for Digital Pathology Solution for Primary Diagnostic Use
Today, Philips announced it has received regulatory clearance from the FDA (via De Novo classification) for its IntelliSite Pathology Solution, marking a significant leap forward for the pathology services industry in the U.S. De novo classification is the regulatory pathway for marketing clearance for novel, low- to moderate-risk medical devices that are the first of their kindAs the first and only digital pathology system to be approved for primary diagnostic use in the country, today’s news
Read More
Philips Acquires Northern Irish Digital Pathology Startup PathXL
Today, Philips announced it has acquired PathXL, Northern Ireland-based leader in digital pathology image analysis, workflow software and educational tools to complement its IntelliSite Pathology Solution. With this move, Philips seeks to accelerate its drive to support medical institutions around the world as they move from microscopy-driven workflows to digitized pathology workflows. Financial details of the acquisition were not disclosed. Founded in 2004 and headquartered in Belfast, Northern
Read More
Massachusetts General Hospital Launches Digital Pathology Study
Massachusetts General Hospital (MGH) announced that it will serve as a key testing site for a clinical analytical study involving Philips’ digital pathology whole slide imaging (WSI) IntelliSite Solution. The study is designed to show the reproducibility of the IntelliSite pathology solution in disease detection as part of the industry’s efforts to obtain regulatory clearance of WSI for primary diagnostic use. If approved for diagnosis, in contrast to the industry’s current analog process, soon
Read More