What You Should Know:
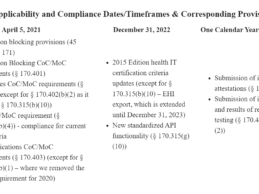
- HHS released an interim final rule to extend compliance dates and timeframes to meet information blocking beyond those identified in April 21, 2020, enforcement discretion announcement and establishes new future applicability dates for information blocking provisions.
- The interim final rule also adopts updated standards
and makes technical corrections and clarifications to the ONC Cures Act Final
Rule.
Today the U.S. Department of
Health and Human Services’
Read More
Health IT Certification
Holon Awarded Patent for Context-Sensing Technology That Surfaces Actionable Patient Data Within Any EHR for Clinicians
Holon announces that it has officially received a US patent for its context-sensing technology that surfaces actionable patient data to providers within any electronic health record (EHR), and from any third-party source, without the need for interfaces. The patented technology platform senses when a provider is in a patient’s chart and automatically surfaces relevant insights, such as care gaps, within the provider’s workflow saving time and reducing administrative burden.Value of
Read More
HHS Proposes New Rule for Enhanced Oversight of Health IT Certification Program
The HHS and ONC today proposed a new rule that would further enhance the safety, reliability, transparency, and accountability of certified health IT for users. The “ONC Health IT Certification Program: Enhanced Oversight and Accountability” proposed rulemaking would modify the ONC Health IT Certification Program to reflect the widespread adoption of certified electronic health records and the rapid pace of innovation in the health IT market.
The proposed rule would focus on three key
Read More