What You Should Know:
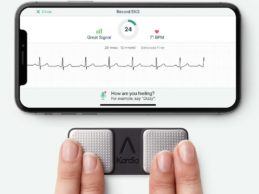
- AliveCor announced they received FDA clearance of new
algorithms for use with their personal EKG devices, KardiaMobile and
KardiaMobile 6L. These additional determinations will be available via a
software upgrade for the Kardia devices in 2021.
- The additional FDA-cleared algorithms double the number
of heart rhythm disturbances that AliveCor’s Kardia devices can detect,
broadening the number of patients who are able to use their remote
Read More
FDA Clearance
FDA Clears First-in-World Hematology App, Unlocking Potential of Diagnosis
What You Should Know:
- Scopio Labs announced FDA clearance of its AI-powered
X100 microscope and decision support system with Full Field Peripheral Blood
Smear (Full Field PBS) application.
- Using advanced computational photography imaging and tailored AI tools, Full Field PBS gives clinical laboratories an unprecedented ability to capture digital scans with full-field view of the monolayer and feathered edge at 100X oil immersion resolution level.
- The global market for
Read More
Tyto Care Launches AI-Powered Diagnostic Support Solution
What You Should Know:
- Today, Tyto Care announced the company’s evolution from
telehealth to tele-diagnosis with its AI-powered diagnostic support solution,
providing clinicians with advanced insights for informed remote diagnoses.
- The solution will improve the quality of remote triage
for common primary and chronic health issues – starting with wheezing and
stridor – grounded in Tyto Care’s industry-leading repository of remote exam
videos, sounds and images.
Tyto Care, a
Read More
Clarius Unveils Ultra-High Frequency Handheld Ultrasound Scanner
What You Should Know:
- A pioneer in high definition ultrasound
miniaturization, Clarius is announcing the new high-frequency handheld
ultrasound scanner Clarius L20 HD featuring the highest definition wireless
imaging for superficial applications.
- Designed to provide extremely high image quality in the near field (skin line to 4cm), the Clarius L20 HD is ideal for a variety of clinical settings including MSK, rheumatology, podiatry, plastic surgery, microsurgery, pediatric
Read More
RADLogics Secures FDA Clearance for AI-Powered Chest X-Ray App for Triage & Prioritization
What You Should Know:
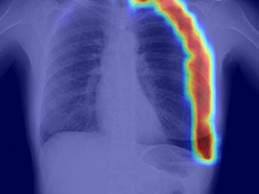
- RADLogics today announced that it has secured
510(k) clearance from the FDA for the company’s novel AI-Powered chest X-ray pneumothorax application,
which identifies and prioritizes chest X-ray scans that appear
to contain a pneumothorax, a collapsed lung, for urgent radiologist review.
- RADLogics’ FDA cleared CT and X-ray solutions
– including this application – are
Read More
Fitbit Awarded Regulatory Clearance in U.S. and Europe for ECG App to Identify AFib
What You Should Know:
- Fitbit has received 510(k) clearance from the
FDA, as well as CE marking in the European Union, for its ECG
app to assess heart rhythm for atrial fibrillation. The Fitbit ECG App is a
simple way people can take an on-the-spot reading of their heart rhythm at any
time, including whenever they notice any unusual cardiac symptoms.
- Fitbit Sense is the company’s first device compatible
with an ECG app that enables users to take a spot
Read More
Caption Health AI Awarded FDA Clearance for Point-of-Care Ejection Fraction Evaluation
What You Should Know:
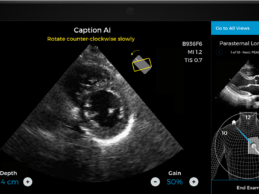
- Caption Health AI is awarded FDA 510(k) clearance for
its innovative point-of-care ejection fraction evaluation.
- Latest AI ultrasound tool makes it even easier to
automatically assess ejection fraction, a key indicator of cardiac function, at
the bedside--including on the front lines of the COVID-19 pandemic.
Caption Health, a Brisbane,
CA-based leader in medical AI technology, today announced it has received FDA
510(k) clearance for an updated version of
Read More
Eko Awarded $2.7M NIH Grant for Heart Murmur & Valvular Heart Disease Detection Algorithms
What You Should Know:
- The National Institutes of Health (NIH) has granted next-generation
cardiac AI company Eko an award totaling $2.7 million to support continued
collaborative work with Northwestern Medicine Bluhm Cardiovascular Institute
- The grant will focus on validating algorithms and help
more accurately screen for heart murmurs and valvular heart disease during
routine office visits with Northwestern Medicine.
- By incorporating data from tens of thousands of
Read More
Activ Surgical Raises $15M Advance Autonomous and Collaborative Surgery
What You Should Know:
- Digital surgery pioneer Activ Surgical raises $15M in funding led by ARTIS Ventures to accelerate U.S. commercialization and European expansion efforts for its ActiveEdge platform, which enables existing surgical systems, from scopes to robots, to visualize, characterize and track tissue, in real-time beyond today’s human capability.
- ActivSight, is an easy-to-adapt, connected imaging module that seamlessly attaches to today’s laparoscopic and arthroscopic
Read More
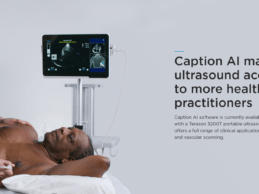
Caption Health Nabs $53M to Commercialize FDA-Cleared AI-Guided Ultrasound Technology
What You Should Know:
- Caption Health raises $53 million series B round to
fuel commercialization and expansion of its FDA-approved AI ultrasound
technology. This is on the heels of its receiving FDA clearance, designated as
a breakthrough technology to fight against COVID-19.
- As ultrasounds, especially cardiac ultrasounds, have
become increasingly important during a time of COVID when resources are
stretched, using AI to help more healthcare professionals take high-quality
images is
Read More