What You Should Know:
- Philips IntelliSite Pathology 5.1 has received 510(k) clearance from the FDA, solidifying its position as the most widely deployed digital pathology solution for primary diagnosis globally.
- With over 300 pathology labs already implementing Philips' solution, and over 20 labs achieving complete digital workflows, the success of this technology is evident.
Investing in the Future of Cancer Care
By empowering collaboration, boosting efficiency, and
Read More
FDA Clearance
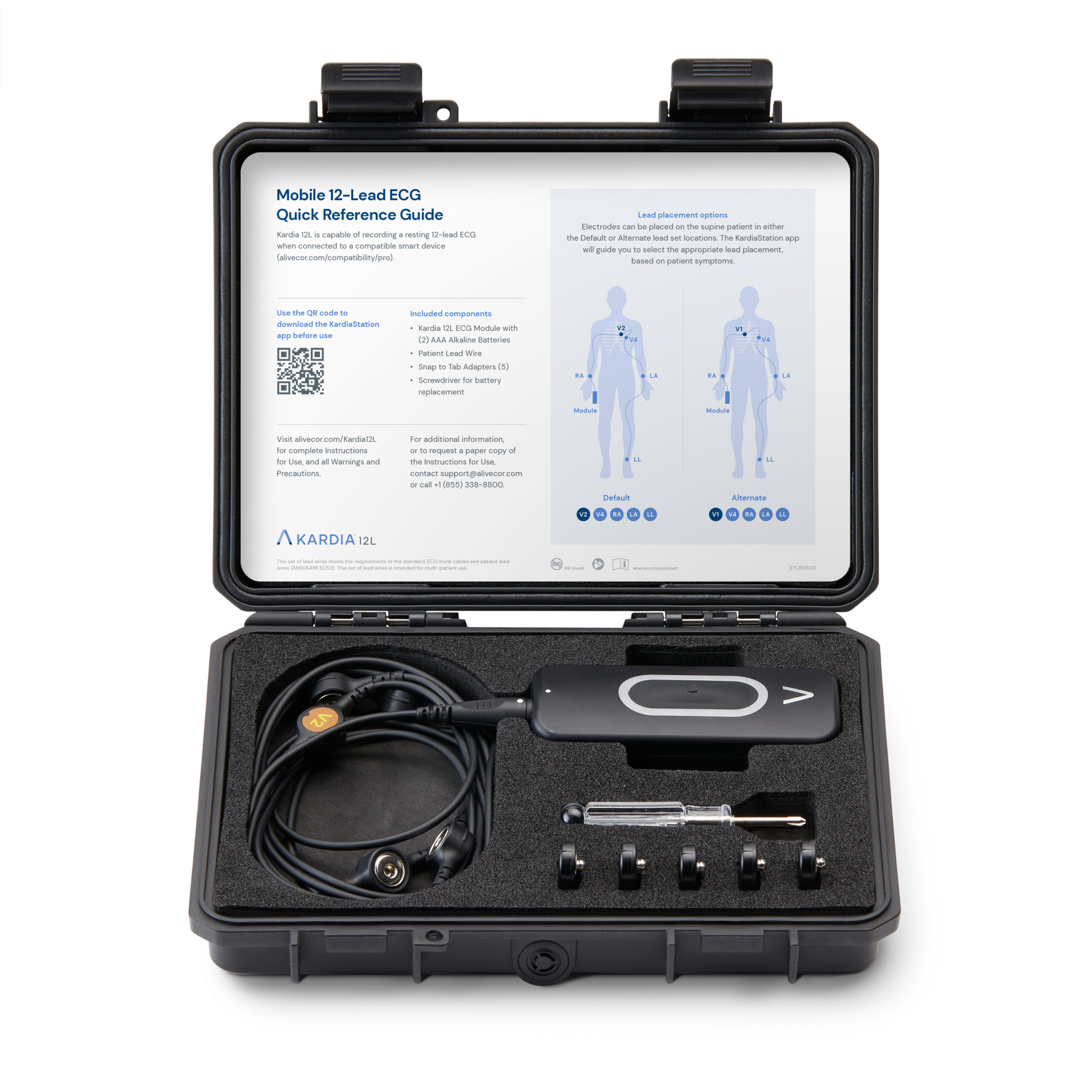
FDA Clears AliveCor’s Heart Attack-Detecting AI Kardia 12L ECG System
What You Should Know:
- AliveCor, a leader in AI-powered cardiology, has received FDA clearance and launched a groundbreaking innovation – the Kardia™ 12L ECG System with KAI™ 12L AI technology.
- AliveCor's Kardia 12L ECG System signifies a major advancement in cardiac diagnostics. By combining cutting-edge AI technology with a portable and user-friendly design, the system empowers healthcare professionals to deliver faster, more accurate, and more accessible cardiac
Read More
Implicity Launches FDA-approved Heart Failure Prediction Algorithm, SignalHF
What You Should Know:
- Implicity, a leader in remote patient monitoring and cardiac data management, has received FDA 510(k) clearance for SignalHF1, a groundbreaking new algorithm within their remote monitoring solution.
- SignalHF1 algorithm empowers early detection and intervention, paving the way for improved patient outcomes and a brighter future for cardiac care.
Leveraging Big Data for Better Heart Care
Implicity holds a unique distinction as the first private
Read More
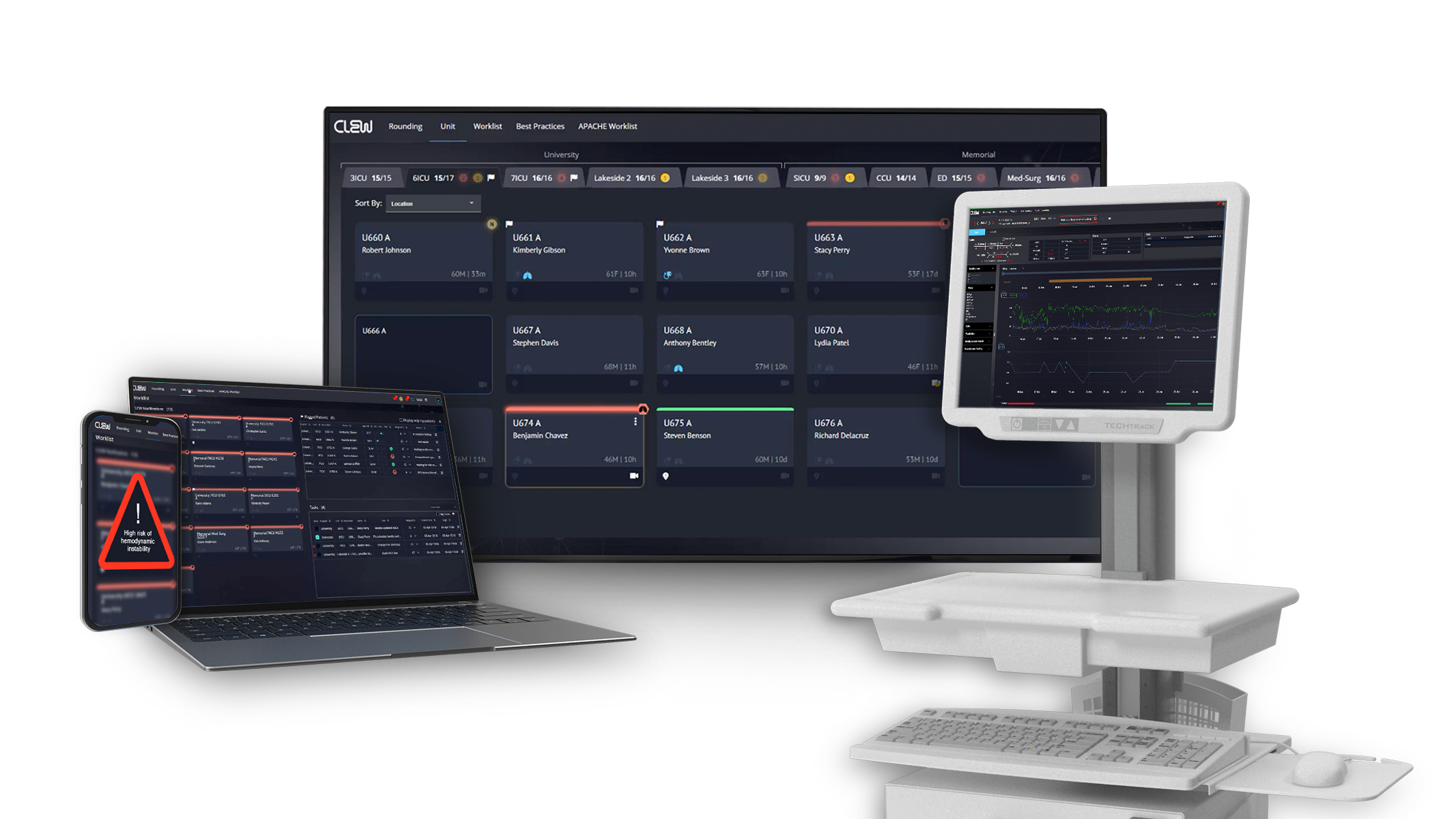
CLEW Medical Scores FDA Clearance for Next-Gen AI-Powered Patient Deterioration Prediction
What You Should Know:
- CLEW Medical, a provider of AI-driven clinical surveillance and predictive analytics, has achieved a breakthrough with FDA 510(k) clearance for its second-generation AI models for predicting patient deterioration.
- The FDA clearance builds upon their pioneering achievement of being the first FDA-cleared Class II medical device for this purpose in 2021.
Rigorous Testing for FDA Approval
Obtaining FDA clearance signifies that CLEW's AI models have
Read More
Exo® Launches FDA-Cleared AI on Exo Iris™ to Address Heart Failure
What You Should Know:
Exo® (pronounced “echo”), a medical imaging software and devices company, today announced its FDA-cleared cardiac and lung artificial intelligence (AI) applications are now available on Exo Iris™, Exo’s high-performance handheld ultrasound device. With affordable, AI-powered medical imaging in a pocket-sized device, caregivers can get to answers immediately to accelerate diagnosis and create new care pathways for heart failure patients at the point of care. As
Read More
FDA Clears First-Ever Digital Bone Marrow Aspirate App
What You Should Know:
- Scopio Labs, a leader in digital cell morphology, has achieved a major breakthrough with FDA De Novo clearance for its Full-Field Bone Marrow Aspirate (FF-BMA) Application.
- The FDA clearance marks a pivotal moment, establishing the first-ever regulatory category for digital bone marrow aspirate analysis software in the US.
Bone Marrow Analysis: Crucial for Diagnosing Blood Disorders
Microscopic analysis of bone marrow samples, known as bone
Read More
Dexcom Earns FDA Clearance for OTC Glucose Biosensor in the U.S.
What You Should Know:
DexCom, Inc. (NASDAQ:DXCM), the global leader in real-time continuous glucose monitoring for people with diabetes, announced today that the FDA has cleared Stelo by Dexcom – the first glucose biosensor that doesn’t require a prescription.There are approximately 25 million people in the U.S. living with Type 2 diabetes who do not use insulin and who can benefit from continuous glucose monitoring (CGM) technology. Today, Dexcom G7 is available for them with a
Read More
X-trodes’ Smart Skin Earns FDA Clearance for Home-Based Electrophysiological Monitoring
What You Should Know:
- X-trodes, a pioneer in wireless monitoring solutions, has achieved a major milestone with the U.S. Food and Drug Administration (FDA) granting 510(k) clearance for its Smart Skin solution, marketed as the X-trodes System M.
- The FDA clearance positions X-trodes as a frontrunner in democratizing advanced electrophysiological monitoring. By bringing clinical-grade technology to the home environment, X-trodes paves the way for personalized healthcare solutions,
Read More
23andMe Scores FDA Clearance for Novel Cancer Immunotherapy Targeting NK Cells
What You Should Know:
- 23andMe Holding Co. (Nasdaq: ME), a genetics and biopharmaceutical company, announced The U.S. Food and Drug Administration (FDA) has cleared the investigational new drug (IND) application for 23ME-01473 (referred to as '1473), paving the way for the first human clinical trial of this promising new therapy.
- With FDA clearance secured, 23andMe plans to initiate a Phase 1 clinical trial in the first half of 2024. This initial study will evaluate the safety and
Read More
AI Breakthrough for Lung Disease: Thirona’s LungQ Receives FDA Clearance
What You Should Know:
- Thirona, a pioneer in AI-powered lung analysis, received a major boost for its cutting-edge technology LungQ™ with the U.S. Food and Drug Administration (FDA) 510(k) clearance for its latest update, v3.0.0.
- This FDA clearance paves the way for American hospitals to now harness the software's advanced capabilities, marking a significant step forward in lung disease diagnosis and treatment.
LungQ 3.0.0: Precision Navigation for Lung Interventions
This latest
Read More