What You Should Know
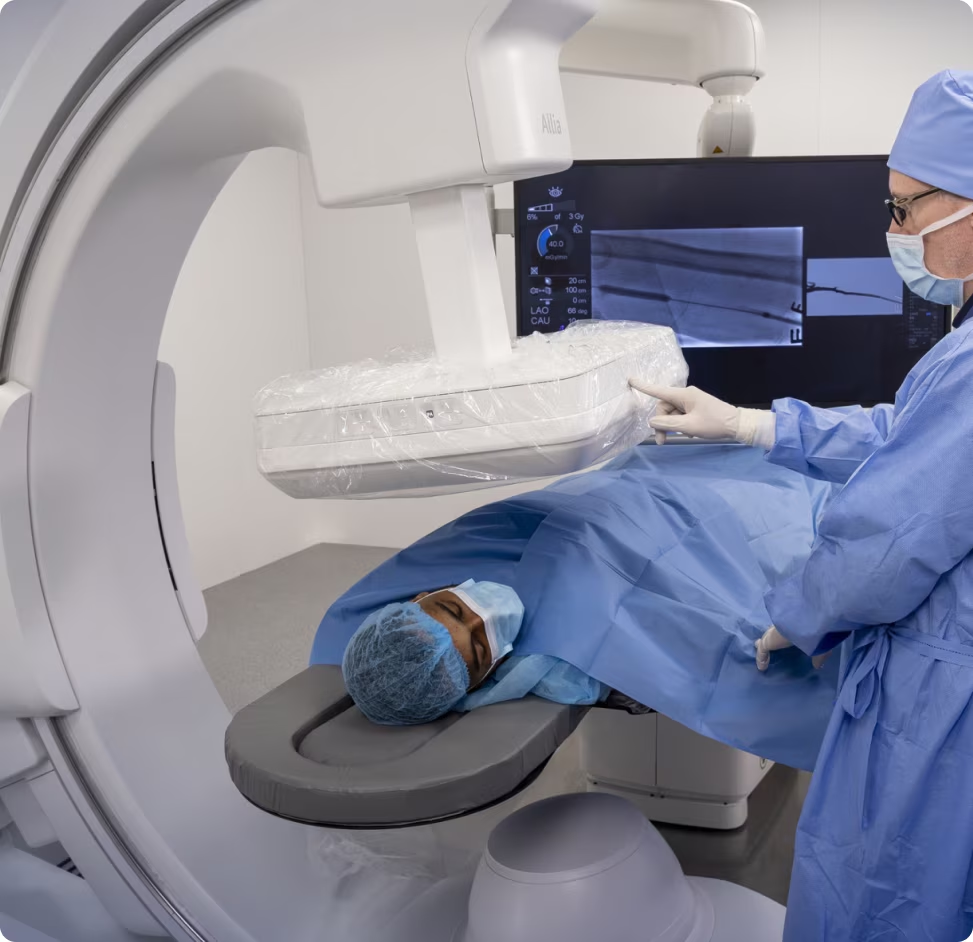
The Launch: GE HealthCare has received FDA 510(k) clearance and CE Marking for Allia™ Moveo, a new mobile C-arm system designed for cardiovascular and interventional procedures.The Design: The system addresses the physical constraints of the operating room. It is compact and cable-free, featuring a "wide-bore" design that accommodates patients of any size while allowing effortless table panning.The AI: The platform integrates CleaRecon DL, an AI-driven tool that removes
Read More
FDA Clearance
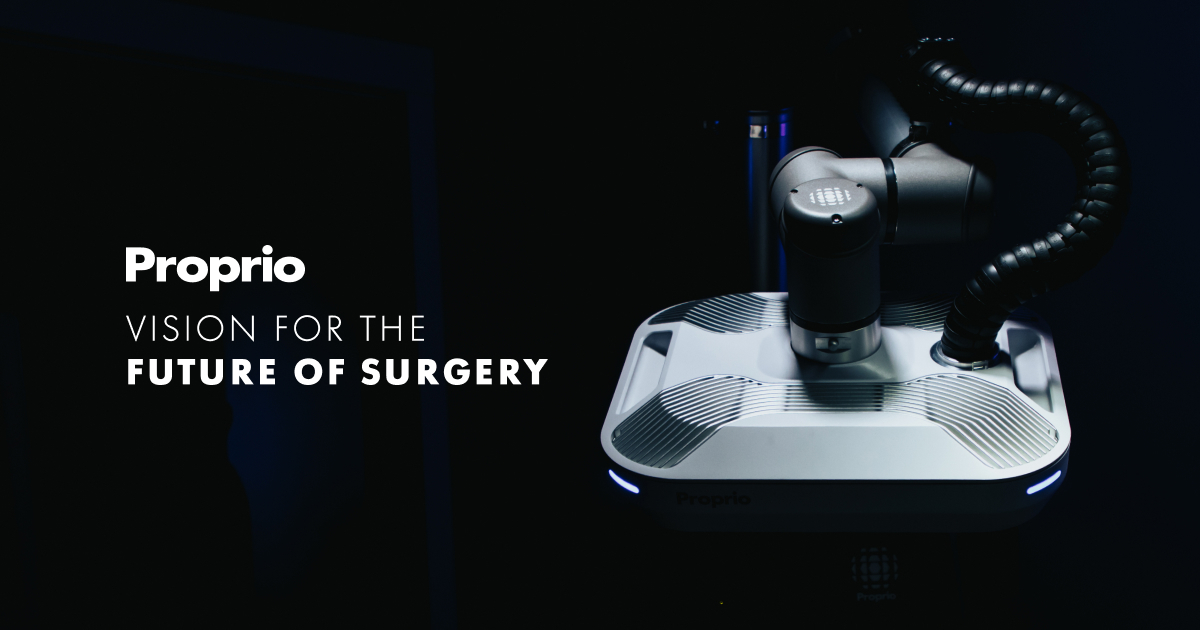
Proprio Secures 4th FDA Clearance for AI-Powered ‘Picasso’ Spine Guidance
What You Should Know:
- Proprio has secured FDA clearance for Picasso, its fourth cleared capability within the Paradigm AI surgical platform.
- The feature enables "trace-based" optical registration, allowing spine surgeons to perform real-time, radiation-free 3D measurements of spinal alignment during surgery—addressing a critical gap where surgeons previously relied on static 2D X-rays or postoperative confirmation.
Radiation-Free "Digital Twins"
Legacy navigation
Read More
AliveCor Receives FDA Clearance for 39 Determinations on Kardia 12L AI
What You Should Know
- AliveCor, the global leader in AI-powered cardiology, has received U.S. FDA clearance for the next generation of its KAI 12L AI. This update expands the diagnostic breadth of the Kardia 12L ECG System to detect five additional cardiac determinations, bringing the platform’s total to 39 cleared determinations.
- The handheld, 0.3-pound device can now identify specific rhythm modifiers and axis-related morphologies, providing clinicians with unprecedented diagnostic
Read More
AccurKardia Secures Second FDA Clearance for AccurECG 2.0 to Tackle Cardiac Backlogs
What You Should Know
- AccurKardia has announced its second FDA 510(k) clearance for the AccurECG™ Analysis System (v2.0). This enterprise-grade, cloud-based platform is designed to ingest data from any ECG hardware—including patches and Holters—and deliver fully automated, near real-time interpretation for 13 rhythm classifications with 99% accuracy.
- As cardiac data volumes surge due to the proliferation of wearables, AccurECG 2.0 allows hospitals and independent diagnostic testing
Read More
RapidAI Secures FDA Clearance for Five New Deep Clinical AI Modules, Expanding Enterprise Imaging Platform
What You Should Know:
- RapidAI, a pioneer in deep clinical AI, announced U.S. FDA clearance for five new imaging modules—Rapid DeltaFuse™, Rapid LMVO, Rapid MLS, Rapid OH, and Rapid Aortic for measurement.
- The expansion reinforces the Rapid Enterprise™ Platform’s focus on bringing deep clinical intelligence and seamless workflow integration across the entire patient journey.
Deep Clinical AI: Shifting Beyond Triage to Full Patient Management
RapidAI’s latest FDA clearances
Read More
Ceribell Receives FDA Clearance for Clarity® AI, Becoming First Point-of-Care EEG for All Ages
What You Should Know:
- Ceribell, Inc. today announced that the U.S. FDA has granted 510(k) clearance for its next-generation Clarity® algorithm.
- The FDA clearance makes the Ceribell System the first and only AI-powered point-of-care electroencephalography (EEG) technology available to detect electrographic seizures in all patients, from pre-term neonates through adults.
Closing a Critical Unmet Need in Neonatal Care
The FDA clearance for the Clarity® algorithm to
Read More
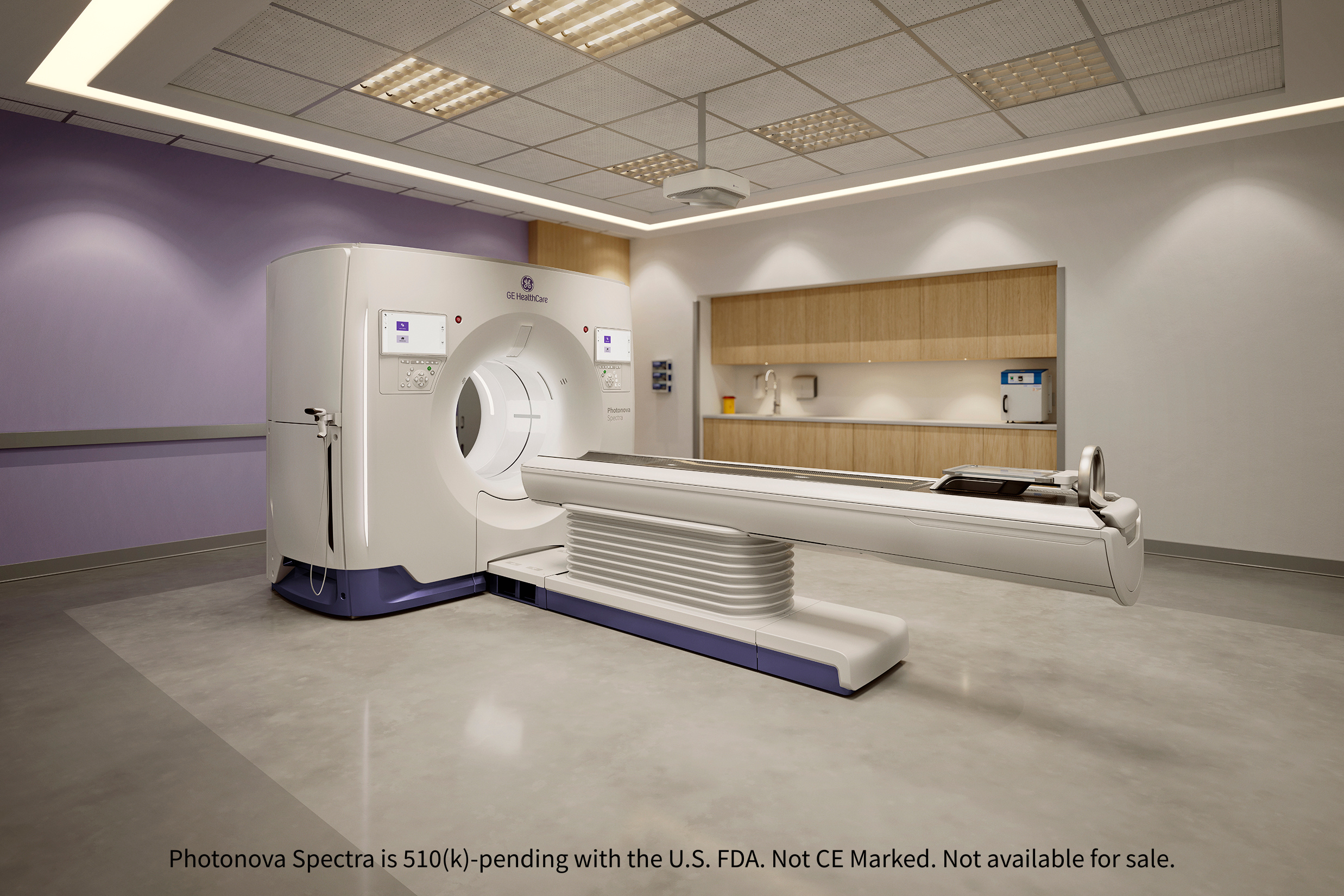
GE HealthCare Submits Photonova™ Spectra for FDA Clearance: Deep Silicon Detectors Power New PCCT System
What You Should Know:
- GE HealthCare has announced the submission of a 510(k) to the U.S. FDA for Photonova™ Spectra, its new photon-counting computed tomography (PCCT) system with advanced AI algorithms.
- Built on proprietary Deep Silicon detector technology, the system aims to redefine diagnostic confidence by delivering ultra-high-definition (UHD) imaging, enhanced material separation, and rapid acquisition speeds.
Photon Counting CT with Deep Silicon
GE HealthCare's
Read More
11 Recent Digital Health FDA Clearances You Need to Know: AI, Wearables, and Robotics Drive Precision Medicine Forward
What You Should Know:
- The U.S. Food and Drug Administration (FDA) has recently issued several key clearances, marking significant advancements across diagnostics, surgery, and remote patient monitoring.
- These FDA clearances reflect a deepening integration of Artificial Intelligence (AI) and advanced technology into clinical workflows, promising greater precision, reduced invasiveness, and expanded access to care.
Diagnostics and Remote Monitoring: AI and Wearables at Scale
A
Read More
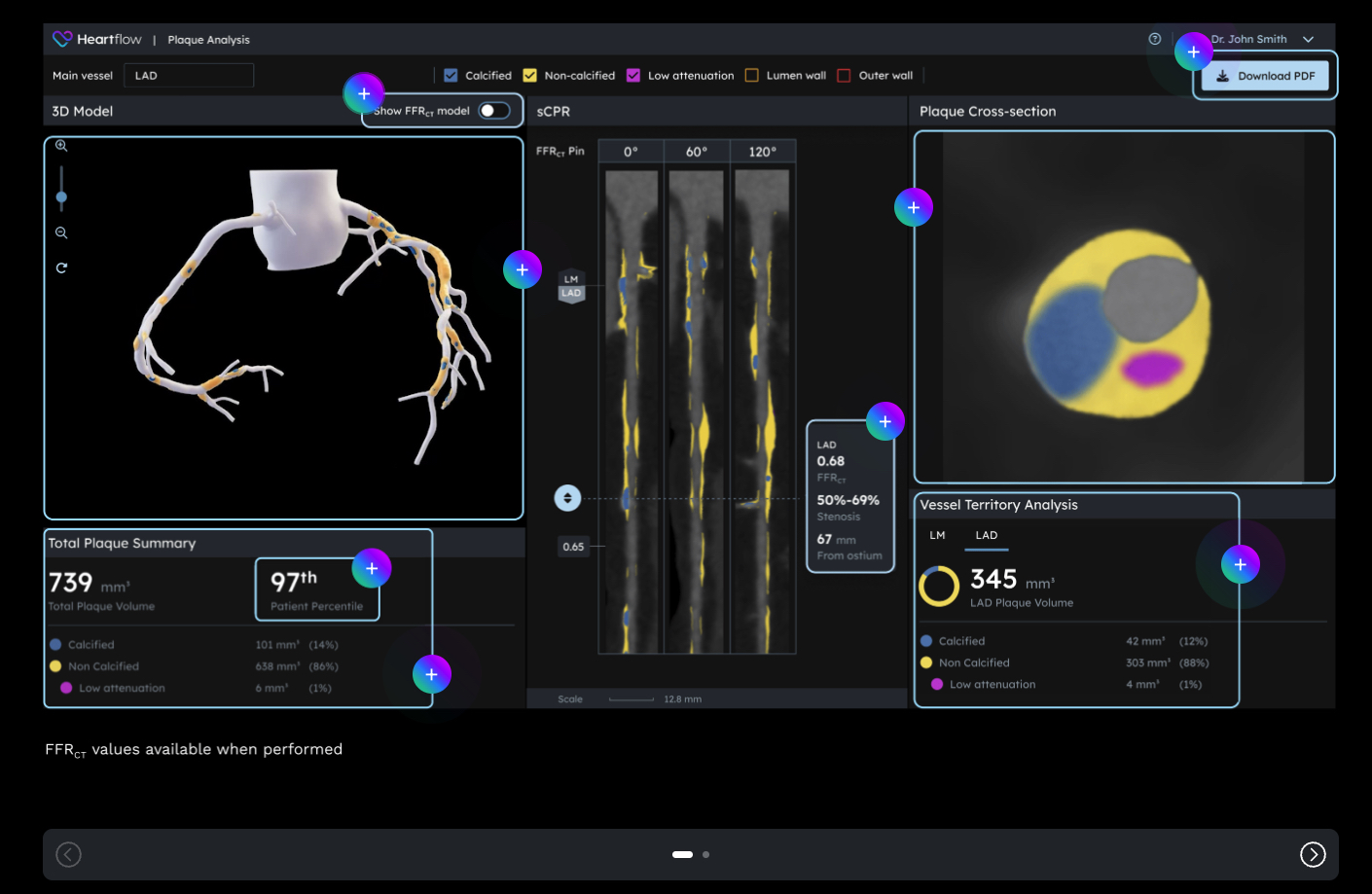
Heartflow Receives FDA Clearance for Next-Gen AI Plaque Analysis, Expands Cigna Coverage
What You Should Know:
- Heartflow, a leader in AI technology for coronary artery disease (CAD), has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Next Gen Heartflow Plaque Analysis algorithm and platform.
- The technology features an updated algorithm, an expanded nomogram, and advanced 3D color-coded visualization of plaque, empowering clinicians with crucial insights for confident care decisions. In addition, Heartflow announced that Cigna will
Read More
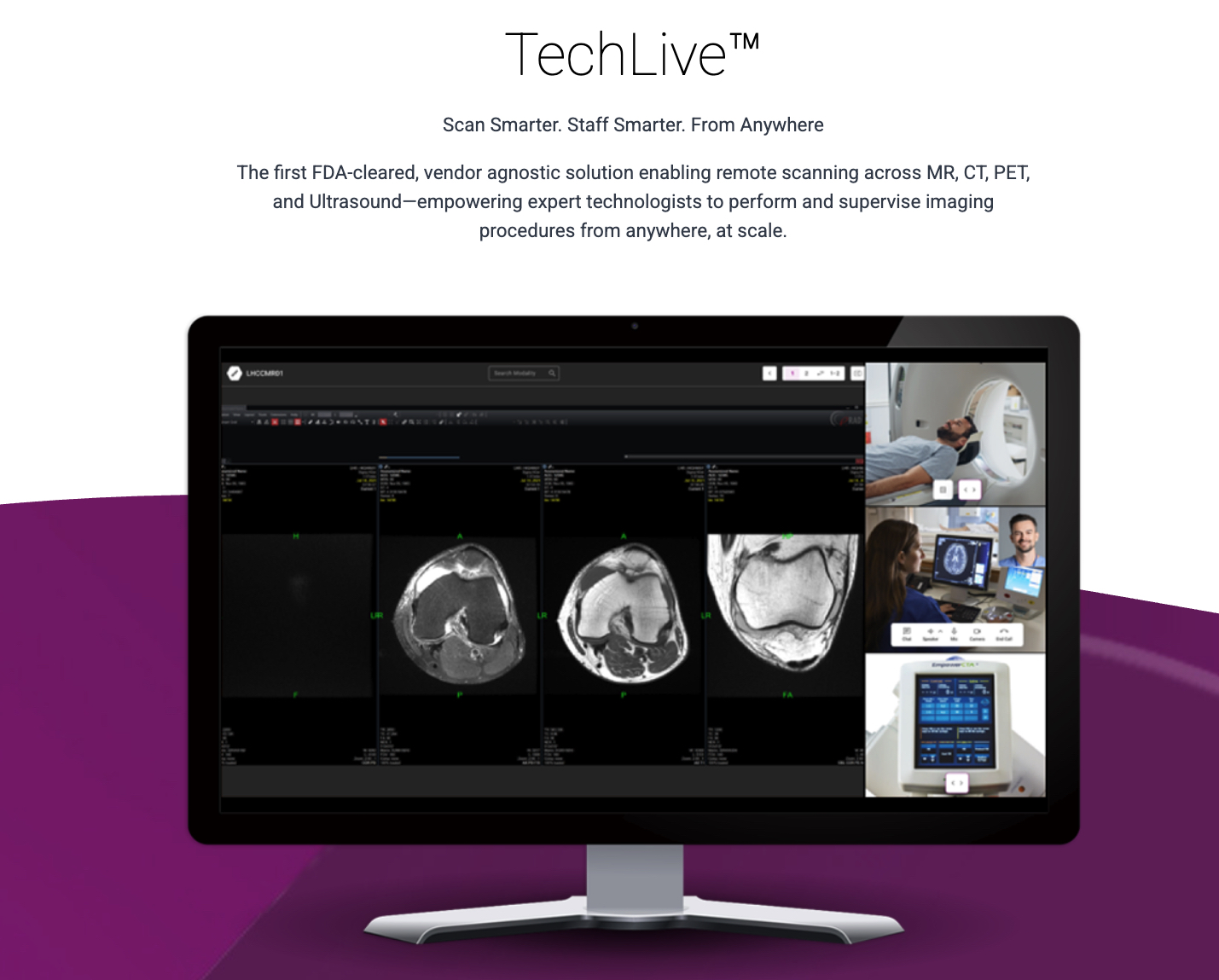
DeepHealth Receives FDA Clearance for Remote Scanning Solution, TechLive™
What You Should Know:
- DeepHealth, a subsidiary of RadNet, has received FDA 510(k) clearance for its new remote scanning solution, TechLive™. The vendor-agnostic platform is designed to address the growing radiology technologist shortage by allowing experts to remotely operate and supervise MR, CT, PET/CT, and Ultrasound procedures from a centralized command center.
- The move comes at a critical time for the healthcare industry, which has seen radiology workloads steadily increase
Read More