What You Should Know:
- Luminopia, a digital health company focused on treating neuro-visual disorders, announced today that Highmark has approved coverage for its FDA-cleared therapy for children with amblyopia.
- This decision marks a significant milestone for Luminopia, as it expands access to its innovative treatment for a condition that affects millions of children.
What is Amblyopia?
Amblyopia, commonly known as lazy eye, is a leading cause of vision loss in
Read More
FuturHealth Combines GLP-1 Medications with Personalized Nutrition
What You Should Know:
- FuturHealth, a leader in personalized weight-loss solutions launches its Custom Meal Plans. Designed to complement the growing popularity of GLP-1 medications, the new offering provides a comprehensive approach to weight management and long-term health.
- As the use of GLP-1 medications surges, FuturHealth recognizes the need for a holistic approach to weight loss. By combining personalized meal plans with medication, FuturHealth aims to help individuals
Read More
Abbott and Medtronic Partner to Integrate CGM System & Insulin Delivery Devices
What You Should Know:
- Abbott and Medtronic announced a groundbreaking partnership to develop an integrated continuous glucose monitoring (CGM) system designed to seamlessly work with Medtronic’s automated insulin delivery (AID) and smart insulin pen systems.
- The strategic collaboration aims to improve diabetes management by enabling more precise insulin dosing and reducing the burden on patients.
CGM System and Insulin Delivery Devices Integration
The new CGM sensor,
Read More
Patients Demand Personalized Obesity Care, New Survey Finds
What You Should Know:
- Phenomix Sciences, a precision medicine biotechnology company, released findings from a new survey revealing a growing demand for personalized obesity care.
- The survey of 475 patients explored attitudes toward weight management, treatment preferences, and information sources.
Key Findings:
Patients Seek Medical Guidance: A majority of respondents (66%) prefer guidance from healthcare providers to manage weight loss, highlighting the need for a
Read More
Physicians Reveals GLP-1s for Weight Loss are Overprescribed and Unsustainable
What You Should Know:
- A large majority of physicians feel GLP-1s are being overprescribed, according to a new survey from Virta Health, a leader in type 2 diabetes reversal.
- The Virta Health survey was conducted in partnership with Beresford Research and included 300 primary care providers, endocrinologists, and obesity specialists across the United States sheds light on the medical community's views on the rapidly growing class of GLP-1 medications, often hailed as
Read More
FemTech: Fertilitywise Partners with Fairfax EggBank to Empower Patients
What You Should Know:
- Fertilitywise, a provider of resources and support for those navigating fertility challenges, announced a new partnership with Fairfax EggBank, a prominent donor egg program. This collaboration aims to enhance the fertility journey for patients by offering comprehensive support and information.
- Through this partnership, Fairfax EggBank patients will receive a complimentary two-month membership to Fertilitywise, providing access to a wealth of evidence-based
Read More
venBio Raises $528M for Fifth Life Sciences Fund
What You Should Know:
- venBio, a leading life sciences venture capital firm, announced the successful closing of its fifth fund, venBio Fund V, with over $528M in capital commitments.
- The new fund will focus on investing in biopharmaceutical companies developing innovative treatments for unmet medical needs.
venBio Fund V Focus
With a strong track record of successful investments in companies such as Labrys Biologics, Aragon Pharmaceuticals, and Seragon
Read More
Spring Health Raises $100M for Mental Health Platform, Reaching $3.3B Valuation
What You Should Know:
- Spring Health, a mental healthcare platform raises $100M in Series E funding round, bringing its valuation to $3.3B. The round was led by Generation Investment Management, with participation from existing investors Kinnevik, William K Warren Foundation, RRE, and Northzone.
- The new funding will enable Spring Health to expand its reach, enhance its technology platform, and deepen its commitment to delivering exceptional mental healthcare. The company plans to
Read More
KONZA Launches Birth Connect to Improve Maternal and Infant Health
What You Should Know:
- KONZA National Network, a leading health information exchange (HIE), announced the launch of Birth Connect, a groundbreaking solution designed to enhance the care of mothers and newborns.
- By providing timely alerts to obstetricians and gynecologists (OB/GYNs) about the birth of their patients' babies, Birth Connect aims to improve maternal and infant health outcomes.
Addressing a Critical Healthcare Challenge
The United States faces a concerning
Read More
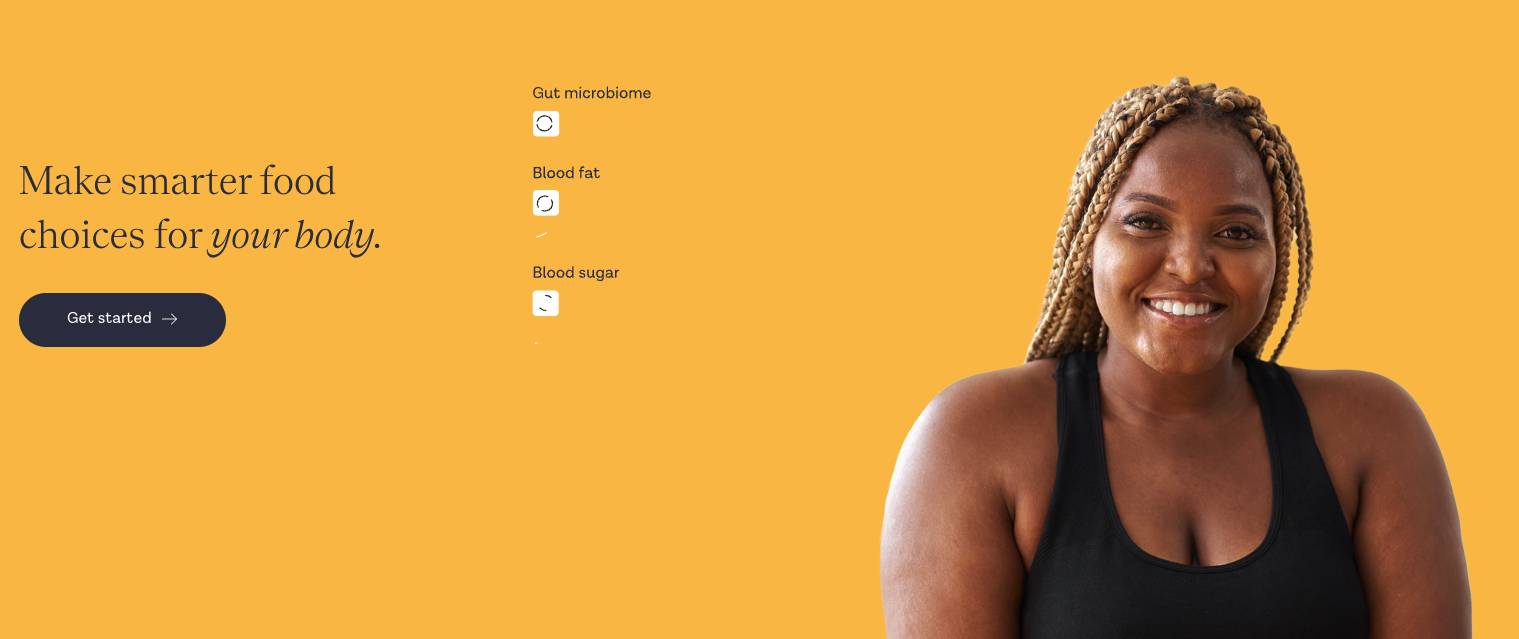
ZOE Secures $15M to Expand US Market and Fight Ultra-Processed Food
What You Should Know:
- ZOE, a science and nutrition company raises $15M from Coefficient Capital to fuel its expansion in the US market.
- The funding will support ZOE’s mission to empower individuals to improve their health through personalized nutrition.
Addressing the Ultra-Processed Food Crisis
ZOE’s science-backed approach challenges the conventional wisdom around nutrition, emphasizing the detrimental impact of ultra-processed foods on long-term health. The
Read More