What You Should Know:
– The U.S. Food and Drug Administration (FDA) has recently issued several key clearances, marking significant advancements across diagnostics, surgery, and remote patient monitoring.
– These FDA clearances reflect a deepening integration of Artificial Intelligence (AI) and advanced technology into clinical workflows, promising greater precision, reduced invasiveness, and expanded access to care.
Diagnostics and Remote Monitoring: AI and Wearables at Scale
A major trend in recent clearances focuses on leveraging AI and comfortable wearables to enhance diagnostic speed and continuity of care.
Wearable Cardiopulmonary Monitoring
Hexoskin Receives 510(k) Clearance for Long-Term ECG and Respiratory Monitoring

Carré Technologies Inc. (dba Hexoskin) received 510(k) clearance for its Hexoskin Medical System (HMS) for continuous long-term ECG, respiratory monitoring, and activity in ambulatory patients. This system, which includes a smart biometric shirt, becomes one of the first medical-grade wearable systems capable of long-term ECG and respiratory measurements outside the clinic, transforming remote care and decentralized clinical trials. The technology enables physicians to assess arrhythmias (like atrial fibrillation) and breathing rate patterns with continuous data collection.
For clinical research, HMS represents a major step forward. With FDA clearance, the system can now support decentralized trials, allowing investigators to capture high-resolution, real-world physiological data and develop AI-driven digital biomarkers across cardiology, pulmonology, neurology, and rare diseases.
AI-Driven Cardiac and Aortic Imaging
FDA clearances solidify AI’s role in cardiovascular risk management:
RapidAI Receives FDA Clearance for Rapid Aortic, Bringing Deep Clinical AI to Aortic Disease Management
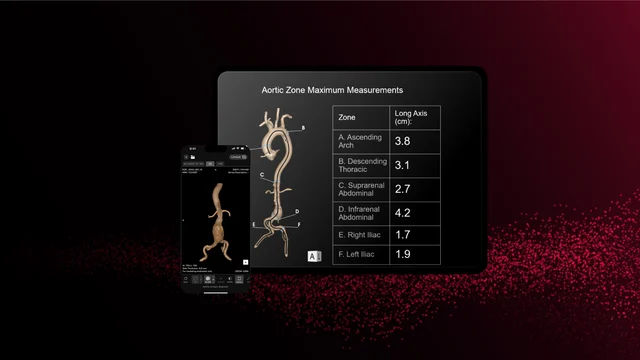
RapidAI earned FDA clearance for Aortic Management, part of its Rapid Aortic product. This deep clinical AI solution transforms the acute assessment and longitudinal management of aortic disease. It automatically generates critical measurements (including zonal maximums and landmark metrics), produces 3D reconstructions, and tracks anatomical changes over time to assist in identifying and tracking pathology from the aortic arch to the iliacs.
Unlike traditional AI triage tools, Rapid Aortic is engineered to support end-to-end patient management: screening, diagnosis, treatment planning, and surveillance. The system’s ability to process all CT scans containing the aorta—whether contrast or non-contrast—expands its utility across emergency, inpatient, and outpatient settings.
Clinicians stand to benefit from reduced cognitive burden, faster read times, and improved accuracy. Surgeons can leverage precise visualizations for pre-procedural planning, while health systems gain a unified workflow integrated through the Rapid Edge Cloud and Rapid Navigator Pro.
Circle CVI Receives 510(k) Clearance for AI-Enabled Coronary Plaque Analysis
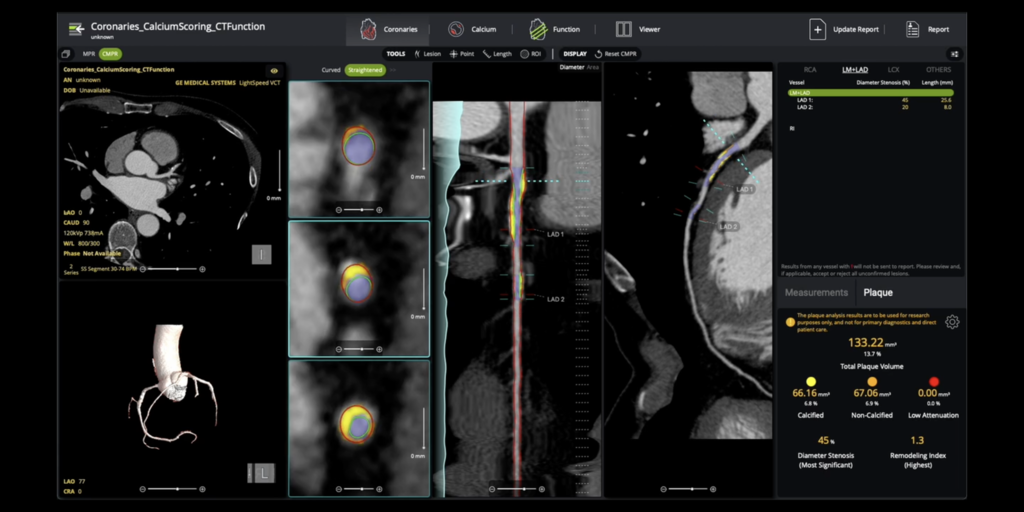
Circle Cardiovascular Imaging Inc. (Circle CVI) received 510(k) clearance for its cvi42 | Plaque solution for comprehensive coronary plaque analysis. This AI-enabled, on-premise technology quantifies total, calcified, and non-calcified plaque, supporting precise risk stratification. The clearance coincides with a new Category I CPT code (75XX6) taking effect in January 2026, solidifying plaque quantification as standard clinical care.
Bunkerhill Health Secures FDA Clearance for First AI to Detect Mitral Annular Calcification on Routine Chest CT

Bunkerhill Health achieved the first-ever FDA clearance for an AI algorithm—Bunkerhill MAC—to detect and quantify mitral annular calcification (MAC) on routine, non-gated chest CT scans. MAC is an often-overlooked finding linked to increased cardiovascular risk and procedural complications.
Integrated into Bunkerhill’s Carebricks platform, the tool leverages FDA-cleared AI and large-language-model-based reasoning to support follow-up decisions within cardiology, primary care, and structural heart programs. The clearance reflects the FDA’s broader confidence in AI that elevates incidental findings into actionable clinical insights.
Neuroscience and Pain Management
Several clearances target brain function and chronic pain:
QuantalX Secures De Novo Clearance for Delphi-MD, a First-of-Its-Kind Functional Neuro-Imaging Technology
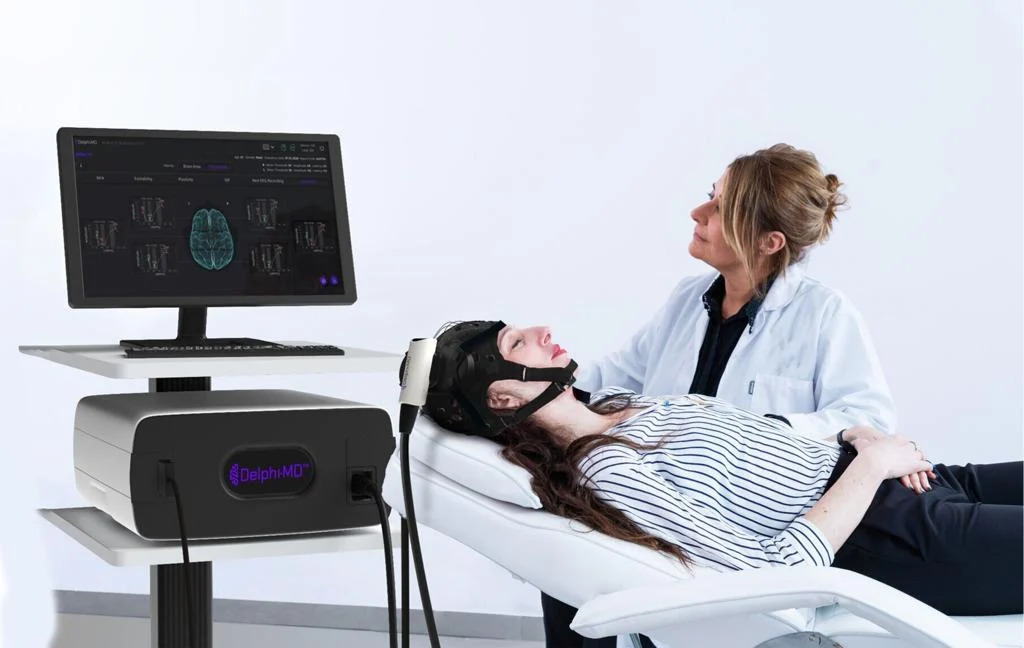
QuantalX Neuroscience was granted De Novo classification for its Delphi-MD™ System, establishing a new modality of functional neuro-imaging (FNI). Delphi-MD combines non-invasive Transcranial Magnetic Stimulation (TMS) with electroencephalography (EEG) to benchmark brain network function against a normative database.
Delphi-MD gives physicians a benchmarked assessment of brain function using an FDA-cleared normative database of healthy adults. This creates a novel clinical modality capable of monitoring cognitive decline, evaluating neurological interventions, and aiding disease management across neurodegenerative, traumatic, or pain-related conditions.
Magstim Achieves FDA Clearance for Non-Invasive Magnetic Stimulation for Chronic Pain
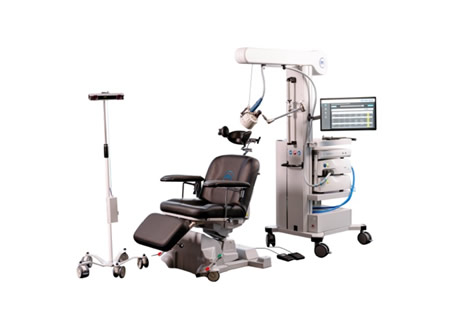
The FDA cleared Magstim Magnetic Stimulation for the treatment of chronic pain, providing a clinically proven, non-invasive, and drug-free option. The technology modulates peripheral nerve pathways using magnetic pulses, reaching deeper nerves without invasive implants or pharmaceuticals.
Magstim’s technology—cited in more than 15,000 scientific studies—provides deeper nerve stimulation compared with traditional TENS or surface-level devices, offering an important option for patients for whom conventional therapies are inadequate.
Surgical Robotics and Precision Orthopedics
Innovations in robotic assistance aim to improve precision and reduce trauma:
Zimmer Biomet Secures 510(k) Clearance for ROSA Knee With OptimiZe, Expanding Robotic Precision in Orthopedics
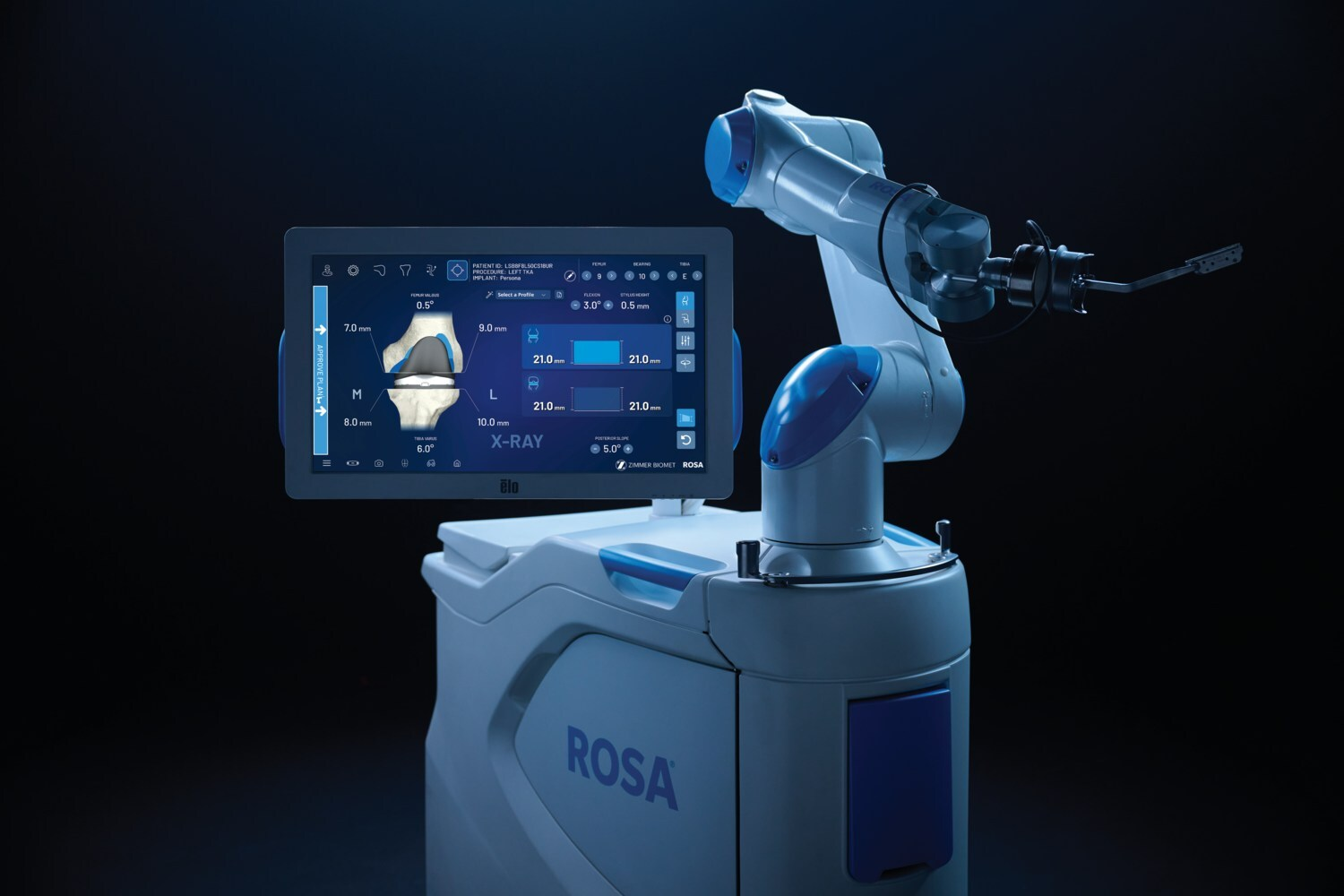
Zimmer Biomet gained 510(k) clearance for ROSA® Knee with OptimiZe™, an enhanced version of its robotic system for total knee replacement surgery. The technology offers customized intelligent surgical planning and features like OptimiZe Kinematic Alignment™—the industry’s only automated kinematic alignment feature—to ensure accurate, reproducible outcomes based on patient anatomy and surgeon preferences.
Integrated with ZBEdge® Analytics, ROSA Knee with OptimiZe enables data-driven decisions, real-time intraoperative insights, and continuous performance evaluation. A targeted release will begin later this year, with U.S. commercial availability expected in early 2026.
Levita Magnetics Gains Pediatric Clearance for Magnetic Surgical System (MSS)
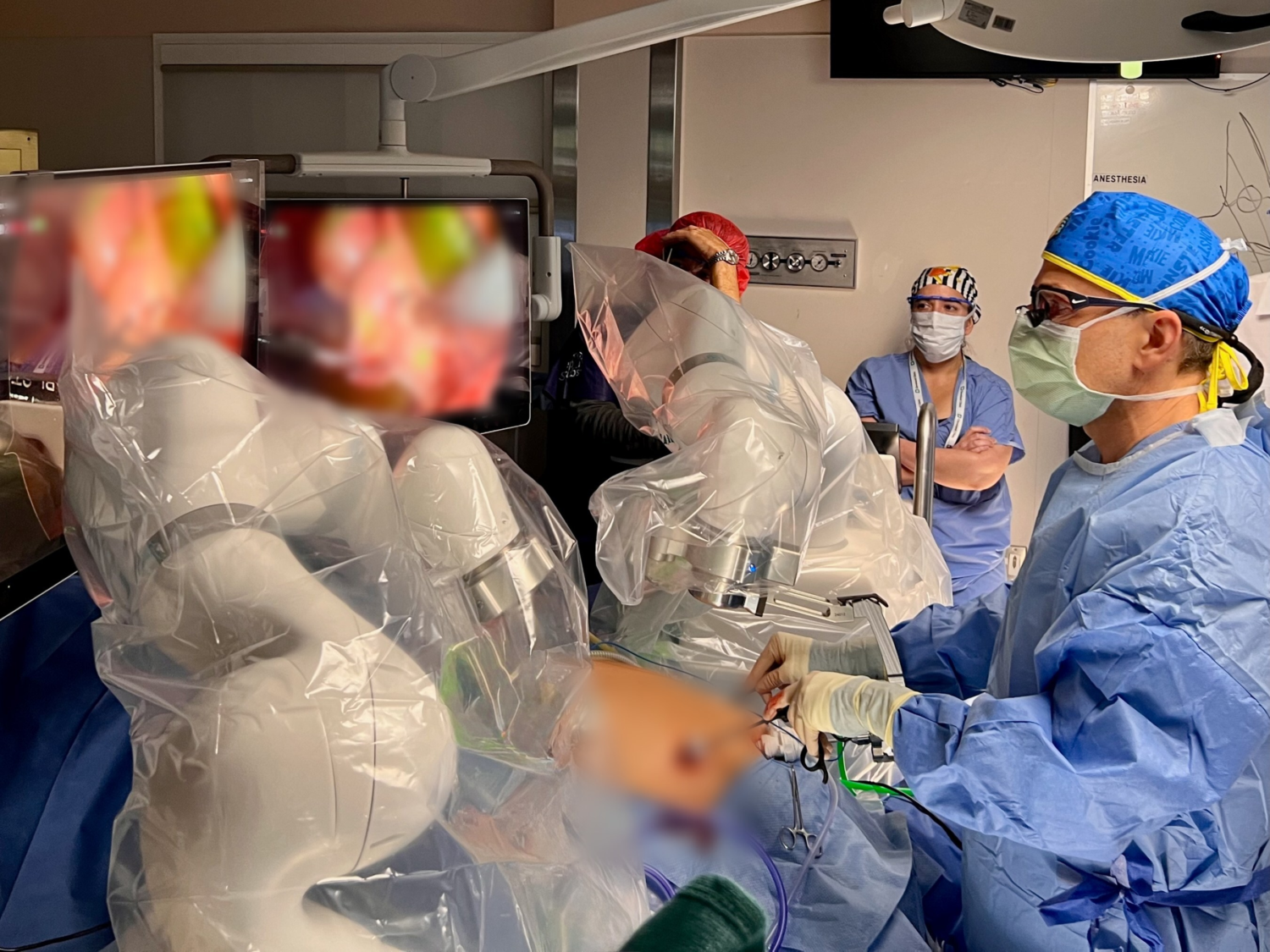
Levita® Magnetics achieved FDA clearance for its Magnetic Surgical System (MSS) for pediatric patients. This technology uses external magnets to control internal surgical instruments, reducing the number of incisions needed for procedures like laparoscopic cholecystectomy, which is critical for minimizing trauma and scarring in children.
Cleveland Clinic Children’s became the first center to perform a pediatric case using this technology. For younger patients, minimizing tissue trauma is critical: fewer ports can lead to faster recovery, decreased pain, reduced scarring, and lower complication risks.
FDA Approves First-Ever Robotic Surgical Study for Alzheimer’s Disease Intervention

MMI (Medical Microinstruments, Inc.) received FDA Investigational Device Exemption (IDE) approval for a clinical study—REMIND—using the Symani® Surgical System for a novel microsurgical intervention for Alzheimer’s disease. The study aims to reestablish lymphatic drainage pathways in the deep cervical lymph nodes to potentially improve the clearance of harmful proteins. The procedure requires supermicrosurgical precision, operating on vessels as small as 0.2mm.
Wound Care and Diabetes Management
Rapid Nexus Earns 510(k) Clearance for Hemastyl, a Breakthrough in Chronic Wound Treatment
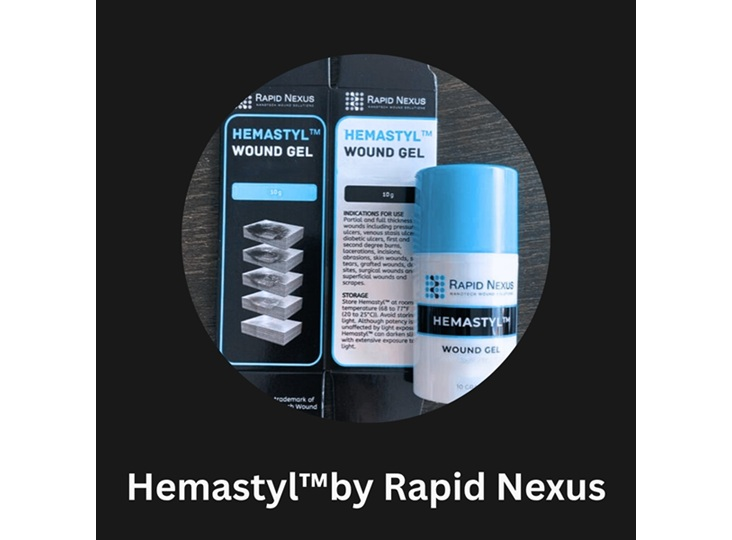
Rapid Nexus Nanotech Wound Solutions, Inc. received FDA 510(k) clearance for its Hemastyl gel device. The gel is the first device to directly treat the periwound tissue environment—the living edge responsible for stalled recovery—to restore conditions for wound closure and help patients avoid amputations.
Rapid Nexus plans to pursue FDA Breakthrough Device Designation, which could expedite coverage and reimbursement pathways, making this therapy available to millions of high-risk patients nationwide. The clearance is a landmark moment for wound healing—a field long dominated by symptomatic rather than causative treatment approaches.
Tandem Diabetes Care Gains FDA Clearance for Tandem Mobi App for Android Users
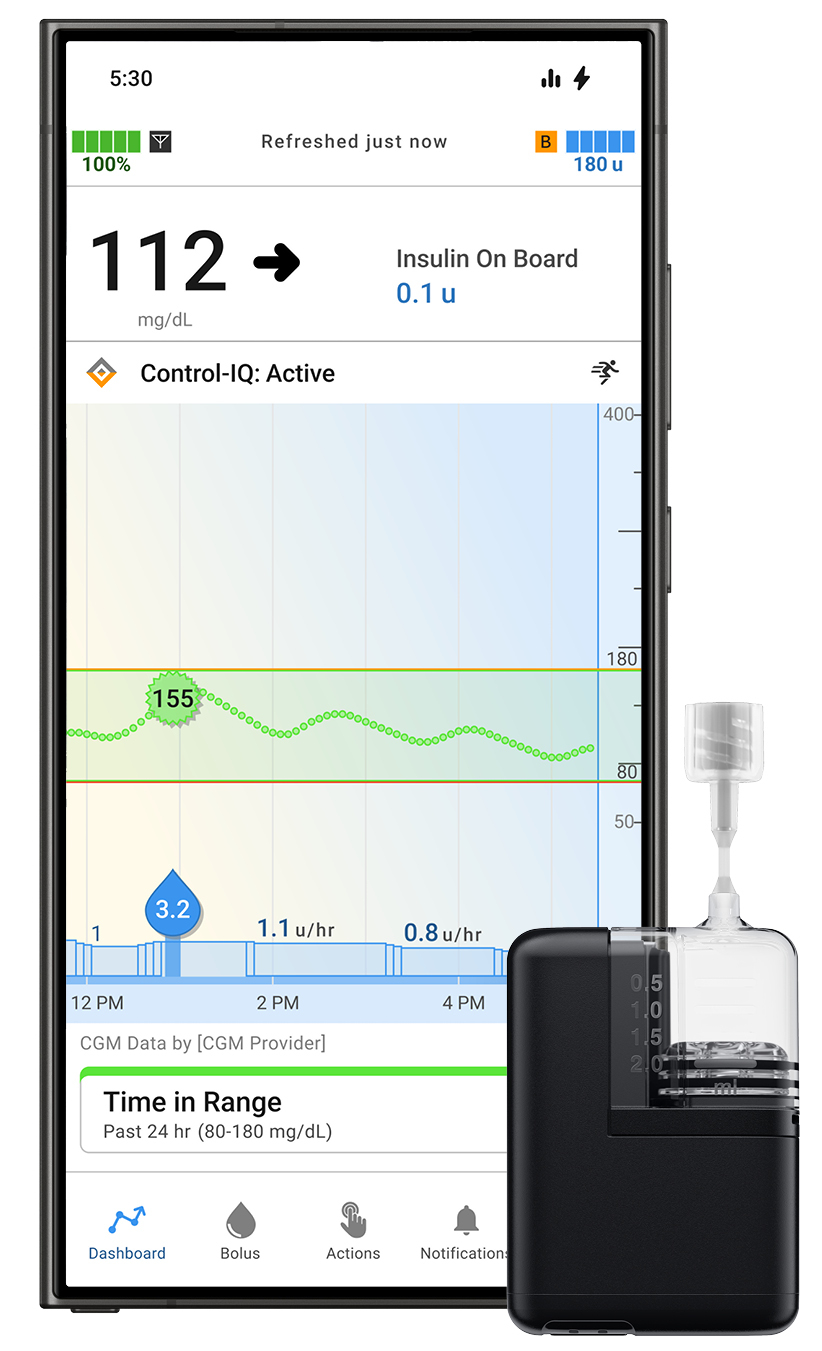
Tandem Diabetes Care, Inc. received FDA clearance for the Android version of its Tandem Mobi mobile app. This allows Android users to manage their diabetes directly from their compatible smartphone using the Tandem Mobi automated insulin delivery system, which is powered by Control-IQ+ technology.
The clearance significantly expands access for patients who prefer Android devices, improving usability and patient adherence. A limited release is expected in late 2025, with broad commercial availability in early 2026.
