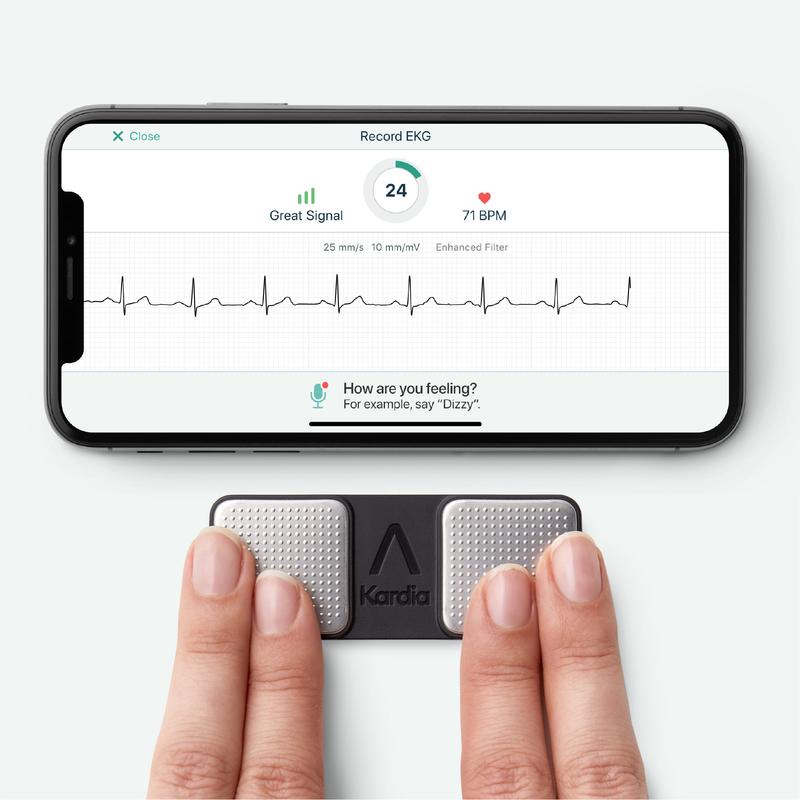
What You Should Know:
– AliveCor announced they received FDA clearance of new algorithms for use with their personal EKG devices, KardiaMobile and KardiaMobile 6L. These additional determinations will be available via a software upgrade for the Kardia devices in 2021.
– The additional FDA-cleared algorithms double the number of heart rhythm disturbances that AliveCor’s Kardia devices can detect, broadening the number of patients who are able to use their remote monitoring devices.
AliveCor, an AI-based personal ECG technology and provider of enterprise cardiology solutions, today announced that the US FDA had given clearance to the company’s next generation of interpretive ECG algorithms. AliveCor’s KardiaMobile and KardiaMobile 6L devices, along with the Kardia app, allow users to take a 30-second ECG and receive instant determinations of multiple cardiac conditions.
Why It Matters
This new FDA clearance positions AliveCor to deliver AI-based remote cardiological services for the vast majority of cases when cardiac patients are not in front of their doctor. AliveCor’s goal is to help cardiologists efficiently provide the best possible 24/7 service to their patients.
New Generation of AI-Powered Remote Cardiology
This new FDA 510(K) clearance provides detail and fidelity unlike any previously seen in personal ECG devices including:
– A “Sinus Rhythm with Premature Ventricular Contractions (PVCs)” determination if two or more ventricular ectopic beats are detected. PVCs are a common occurrence where extra heartbeats originate in the bottom chamber of the heart and occur sooner than the next expected regular heartbeat. After the PVC beat, a pause usually occurs, which causes the next normal heartbeat to be more forceful. When one feels the heart “skip a beat,” it is this more forceful beat that is felt.
– A “Sinus Rhythm with Supraventricular Ectopy (SVE)” determination if narrow-complex ectopy, such as premature atrial contractions (PACs), are detected. PACs are similar to PVCs, but these beats originate in the top chamber of the heart, however not in the heart’s natural pacemaker, the Sinus Node.
– A “Sinus Rhythm with Wide QRS,” determination for QRS intervals of 120ms or longer. Wide QRS indicates that the activation of the bottom chamber of the heart is taking longer than expected. This could indicate a bundle branch block in which there is a delay in the passage of heart’s electrical signals along the bottom of the heart.
– A reduced number of “Unclassified” readings, thereby giving users more reliable insight into their heart rhythms.
– Improved sensitivity and specificity on the company’s “Normal” and “Atrial Fibrillation” algorithms, giving users fewer false positives, fewer false negatives, and even greater confidence in Kardia determinations.
– New visualizations, including average beat, PVC identification, and a tachogram.
“Kardia AI V2 is the most sophisticated AI ever brought to personal ECG,” said AliveCor CEO Priya Abani. “This suite of algorithms and visualizations will provide the platform for delivery of new consumer and professional service offerings beyond AFib, by allowing a much wider range of cardiac conditions to be determined on a personal ECG device.”
Availability
Today, KardiaMobile and KardiaMobile 6L are the most clinically validated personal ECG devices in the world, and provide instant detection of Normal Sinus Rhythm, Atrial Fibrillation, Bradycardia, and Tachycardia. The new determinations and services will be available in 2021.
