DreaMed Diabetes, an Israeli-based developer of personalized diabetes management solutions, today announced that the U.S Food and Drug Administration (FDA) has granted a De Novo request for DreaMed Advisor Pro, an artificial intelligence (AI)-based diabetes treatment decision support software. Advisor Pro is indicated to assist healthcare providers in the management of people with type 1 diabetes who use insulin pumps and continuous glucose monitoring (CGM).
The announcement marks the second regulatory approval for Advisor Pro this year, having received the EU CE Mark in February 2018. In addition, DreaMed received CE Mark for its artificial pancreas technology in 2015, Glucositter, which was licensed by global health technology leader Medtronic Diabetes.
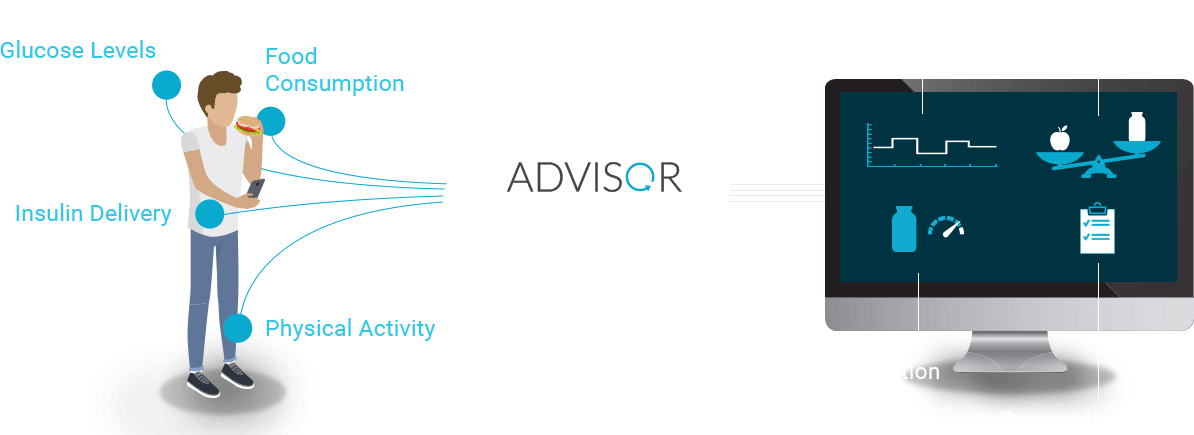
DreaMed Advisor Pro Overview
DreaMed Advisor Pro is a cloud-based digital solution generating insulin delivery recommendations by analyzing information from CGM, self-monitoring blood glucose (SMBG), and insulin pump data. Applying event-driven adaptive learning, Advisor Pro refines its understanding for each individual and sends recommendations to the healthcare provider on how to optimize a patient’s insulin pump settings for basal rate, carbohydrate ratio (CR) and correction factor (CF).
Advisor automatically adjusts its insulin treatment and behavior modification recommendations on a personal and individual basis. Working as a healthcare provider’s “expert partner”, Advisor offers unique insight and direction consistently, quickly and on-demand.
Key benefits for people with diabetes include:
– Treatment plans tailored to your lifestyle
– Guidance for systematic self-management
– Increased access to diabetes expertise and remote consultations
– Less time spent at the doctor’s office
– Fast and accurate insulin adjustments that won’t disrupt your life
– Easy to understand reporting
– Improved glucose control
“This is an innovation that can improve people’s lives and the FDA decision confirms what we believe is an important step in making a more meaningful connection between the healthcare provider and their type 1 diabetes patients,” said DreaMed CEO Eran Atlas. “Type 1 diabetes, managed with greater attention, leads to improved patient quality of life and reduced payer health-related costs. Advisor Pro enables patients using CGM and an insulin pump to analyze data and recommend to their provider when a change in diabetes-care treatment is timely and needed.”
There are more than one million people in the United States with type 1 diabetes. According to the T1D Exchange, the largest clinic registry of patients with type 1 diabetes in the U.S., about 50% of patients in the registry use insulin pumps. This added digital health capability is a step toward making pump and CGM technology more valuable in diabetes management.
Two years ago, in anticipation of the FDA review, DreaMed and Glooko, the leader in diabetes data management, signed an agreement that enables Advisor Pro to be integrated into the Glooko diabetes data management platform.
“This is a significant achievement and practical example of how AI and digital health can improve patient care and enable care teams,” said Russ Johannesson, CEO of Glooko. “We congratulate DreaMed and look forward to working closely with them to demonstrate that Advisor Pro can play a central role in optimizing insulin therapy.”
“Today marks a milestone for people living with type 1 diabetes (T1D). The FDA clearance reinforces why Helmsley supports the development of technologies like DreaMed Advisor Pro that show promise for improving lives in the T1D community,” said David Panzirer, a Trustee of The Leona M. and Harry B. Helmsley Charitable Trust, which in 2016 awarded a $3.4M grant in support of Advisor Pro. “Managing T1D can be overwhelming both for people living with it, and for their clinicians. The DreaMed Advisor Pro harnesses the power of AI to optimize insulin regimens, and will undoubtedly lead to better outcomes for people living with T1D – a driving vision here at Helmsley.”
