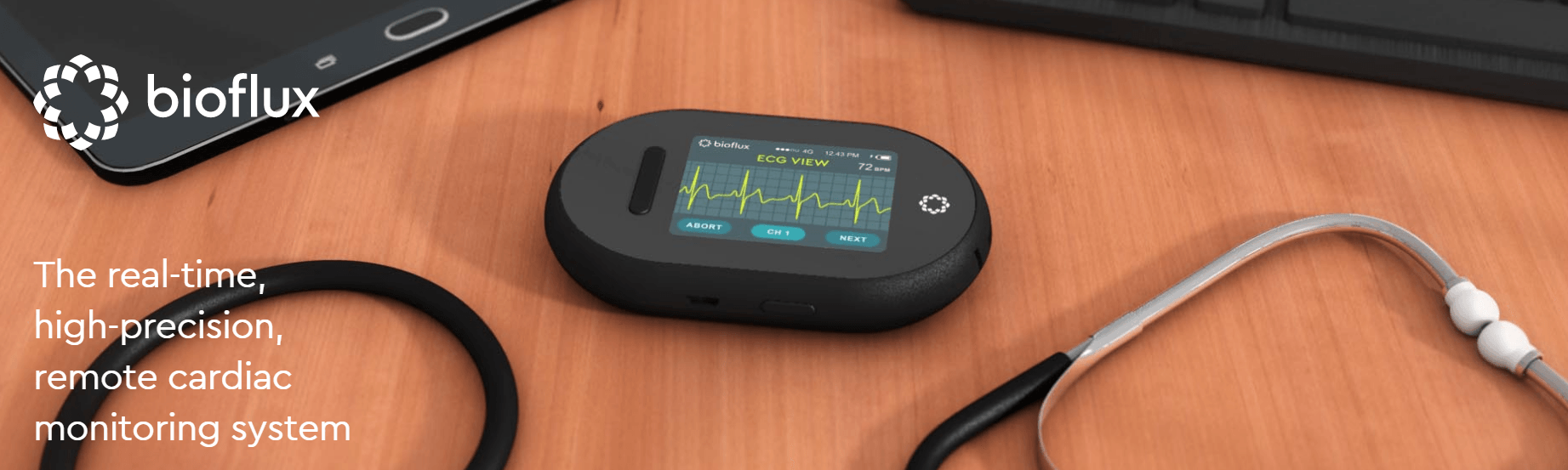
biotricity, a Redwood City, CA-based healthcare technology company has filed for a second and final 510(k) for the hardware portion of its remote cardiac monitoring software and device, Bioflux solution with the U.S. Food and Drug Administration (FDA). The announcement follows a previous milestone where Biotricity received FDA approval for the software portion of its remote cardiac monitoring wearable.
This 510(k) is the final regulatory requirement needed for Biotricity to bring its Bioflux solution to the multi-billion dollar cardiac monitoring market. Biotricity’s flagship product, the Bioflux solution, is designed to combine a proprietary mobile ECG monitoring device and an ECG viewer software package. With 510(k) clearance, the combination would enable physicians to remotely monitor and diagnose patients with cardiovascular disease and coronary heart disease by detecting arrhythmias, using an accredited 24 hour, 7 day per week, ECG monitoring facility.
Upon commercial availability, the device will be used by physicians and hospitals in the diagnostic process and then by patients for long-term care management. This is particularly important because traditional healthcare diagnostic solutions are generally restricted to the physician’s office or inside of the hospital. bioflux’s software is designed to integrate seamlessly into a physician’s practice and workflow with no changes to internal processes.
Available only by subscription, bioflux will monitor a patient’s heart rhythm and send data in real-time to a 24-hour monitoring lab. If a patient is in cardiac distress, an alert will be sent to the monitoring center, which will in turn contact the patient to offer assistance.
“The company is incredibly excited about reaching this key milestone. Submitting a 510(k) for our hardware is a very important step for the company as we prepare to commercialize our first medical solution. We believe significant opportunity exists for our remote patient monitoring solutions to gain traction in the rapidly expanding diagnostic and preventative healthcare markets,” said Biotricity founder and CEO Waqaas Al-Siddiq.
