Overview of the CMS/ONC recently released meaningful use stage 2 final rule requirements for the Electronic Health Records incentive programs
* UPDATE 8/27/12
HIMSS Statement on Release of Meaningful Use Stage 2 and Standards & Certification Criteria Final Rules
In our initial review of the Medicare and Medicaid Programs; Electronic Health Record Incentive Program–Stage 2 Final Rule from the Centers for Medicare and Medicaid, HIMSS has identified several significant policy decisions, including:
- Setting the Meaningful Use Stage 2 start date as 2014, which will maximize the number of eligible professionals (EPs), eligible hospitals (EHs), and critical access hospitals (CAHs) prepared to meet Stage 2 requirements
- Allowing a 90-day reporting period in Year 1 of Stage 2, which is consistent with HIMSS’ recommendations on the proposed rule
- Accepting 2013 as the attestation deadline for EPs, EHs, and CAHs to avoid a Medicare payment adjustment, and allowing for exceptions, including limited availability of information technology
- Finalizing Clinical Quality Measure submission specifications for EPs, EHs, and CAHs
Today, the ONC and CMS have released the final requirements for Stage 2 Electronic Health Records Incentive Program. The final rules specify the Stage 2 criteria eligible professionals (EPs), eligible hospitals, and critical access hospitals (CAHs) must meet in order to continue to participate in the EHR Incentive Programs. The following ONC table is an overview of EHR certification criteria required to satisfy the base EHR definition:
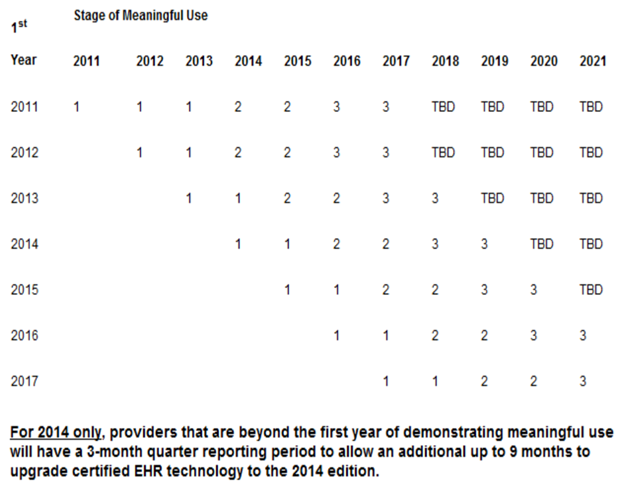
In the fact sheet, CMS states that the stage 2 final rule will give healthcare providers more time to meet the Stage 2 criteria with a provider who has attested for Stage 1 in 2011 would attest to Stage 2 in 2014. This is great news as providers are NOT required to meet Stage 2 meaningful use before 2014. CMS has provided the following table shown below that illustrates the progression of meaningful use stages from the first year a Medicare provider begins in the program.
The 2014 final rule reflects the ONC’s goals to:
- Reduce regulatory burden
- Enhance EHR’s technology interoperability
- Promote patient safety and patient engagement
- Electronic health information exchange capacity
- Public Health Reporting
- Security
- Enable clinical quality measure data capture, calculation, and electronic submission to CMS or states
- Introduce greater transparency and efficiency to the certification process
Key highlights for Stage 2 Meaningful Use Final Rule include:
- The final rule adds “outpatient lab reporting” to the menu for hospitals and “recording clinical notes” as a menu objective for both EPs and hospitals.
- There will be 20 measures for EPs (17 core and 3 of 6 menu) and 19 measures for eligible hospitals and CAHs (16 core and 3 of 6 menu).
- Final rule reduces some thresholds for achieving certain measures and modifies criteria for exclusions to respond to difficulties commenters identified in implementing certain objectives in certain situations.
New Core Objectives:
- New EP Stage 2 Core Objective: Use secure electronic messaging to communicate with patients on relevant health information. (See “Patient Engagement” section below for additional information.)
- New Eligible Hospital/CAH Stage 2 Core Objective: Automatically track medications from order to administration using assistive technologies in conjunction with an electronic medication administration record (eMAR).
- Group Reporting– Ability to use bath reporting process for meaningful use
- Patient Engagement – Two new core objectives (providing patients online access to health information; secure messaging between patient and provider) with measures that require patients to take specific actions in order for a provider to achieve meaningful use and receive an EHR incentive payment.
- Electronic Exchange of Summary of Care Documents – In the final rule CMS is reducing the first measure to a lower threshold of 50 percent. The second measure required that a provider electronically transmit a summary of care for more than 10 percent of transitions of care and referrals, and that the summary of care be electronically sent to a provider with no organizational or vendor affiliation.
- Outpatient Lab Reporting for Hospitals – Rule includes lab reporting as a menu objective that gives hospitals the flexibility to select other objectives for meeting MU and receiving the incentive payment.
- Hospital-based EP Definition – CMS has modified the regulations on “hospital based” so that EPs who can demonstrate that they fund the acquisitions, implementation, and maintenance of CEHRT, including supporting hardware and interfaces needed for meaningful use without reimbursement from an eligible hospital or CAH —and use such CEHRT at a hospital, in lieu of using the hospital’s CEHRT—can be determined non-hospital based and receive an incentive payment.
Clinical Quality Measures (CQMs)
- EPs must report on 9 out of 64 total clinical quality measures (CQMs)
- Eligible hospitals and CAHs must report on 16 out of 29 total CQMs
All providers must select CQMs from at least 3 of the 6 key health care policy domains from the Department of Health and Human Services’ National Quality Strategy:
- Patient and Family Engagement
- Patient Safety
- Care Coordination
- Population and Public Health
- Efficient Use of Healthcare Resources
- Clinical Processes/Effectiveness
Data Submission
Beginning in 2014, all Medicare providers that are beyond the first year of demonstrating meaningful use must electronically report their CQM data to CMS.
EPs can electronically report CQMs either individually or as a group using the following methods:
- Physician Quality Reporting System (PQRS)—Electronic submission of samples of patient-level data. EPs can also report as group using the PQRS GPRO tool. EPs that are beyond the first year of demonstrating meaningful use who electronically report using this PQRS option will meet both their EHR Incentive Program and PQRS reporting requirements.
- CMS Portal—Electronic submission of aggregate-level data.
For more information on MU Stage 2 final rule can be found and downloaded at:
- · CMS – http://www.ofr.gov/OFRUpload/OFRData/2012-21050_PI.pdf
- · ONC – http://www.ofr.gov/OFRUpload/OFRData/2012-20982_PI.pdf
A fact sheet on CMS’s final rule is available at http://www.cms.gov/apps/media/fact_sheets.asp.
A fact sheet on ONC’s standards and certification criteria final rule is available at http://www.healthit.gov/policy-researchers-implementers/meaningful-use-stage-2-0.