What You Should Know
- ConcertAI and Foundation Medicine have announced a major strategic collaboration to integrate their massive data assets, creating a dataset of nearly half a million patients.
- By pairing Foundation Medicine’s de-identified multimodal genomic testing data with ConcertAI’s high-quality clinical records, the partnership provides life science researchers with the market's most comprehensive view of the cancer patient journey—from pre-diagnosis to long-term
Read More
Real World Evidence (RWE)
Verana Health Acquires COTA to Build Multi-Specialty RWD Powerhouse
What You Should Know:
- Verana Health®, a digital health company dedicated to revolutionizing patient care and clinical research through real-world data (RWD), has announced the merger with COTA Healthcare®.
- The merger expands the company’s therapeutic offerings in data and software solutions for real-world insights that accelerate clinical research, expedite treatment options, and improve patient care. Terms of the transaction were not disclosed.
Building a Multi-Specialty Engine for
Read More
Unlocking Real-World Evidence: 5 Questions to Ask Before Trusting AI-Curated RWD
In today’s pharmaceutical industry, real-world evidence (RWE) offers significant potential across all phases of the product life cycle, from trial design to product launch, from pricing and competitive reviews to evaluating the effects of switching medications.
As RWE becomes a key tool for life sciences organizations to shape clinical trial strategies, advance treatment development, and meet regulatory requirements, the quality of the underlying data and trust in how it was generated is
Read More
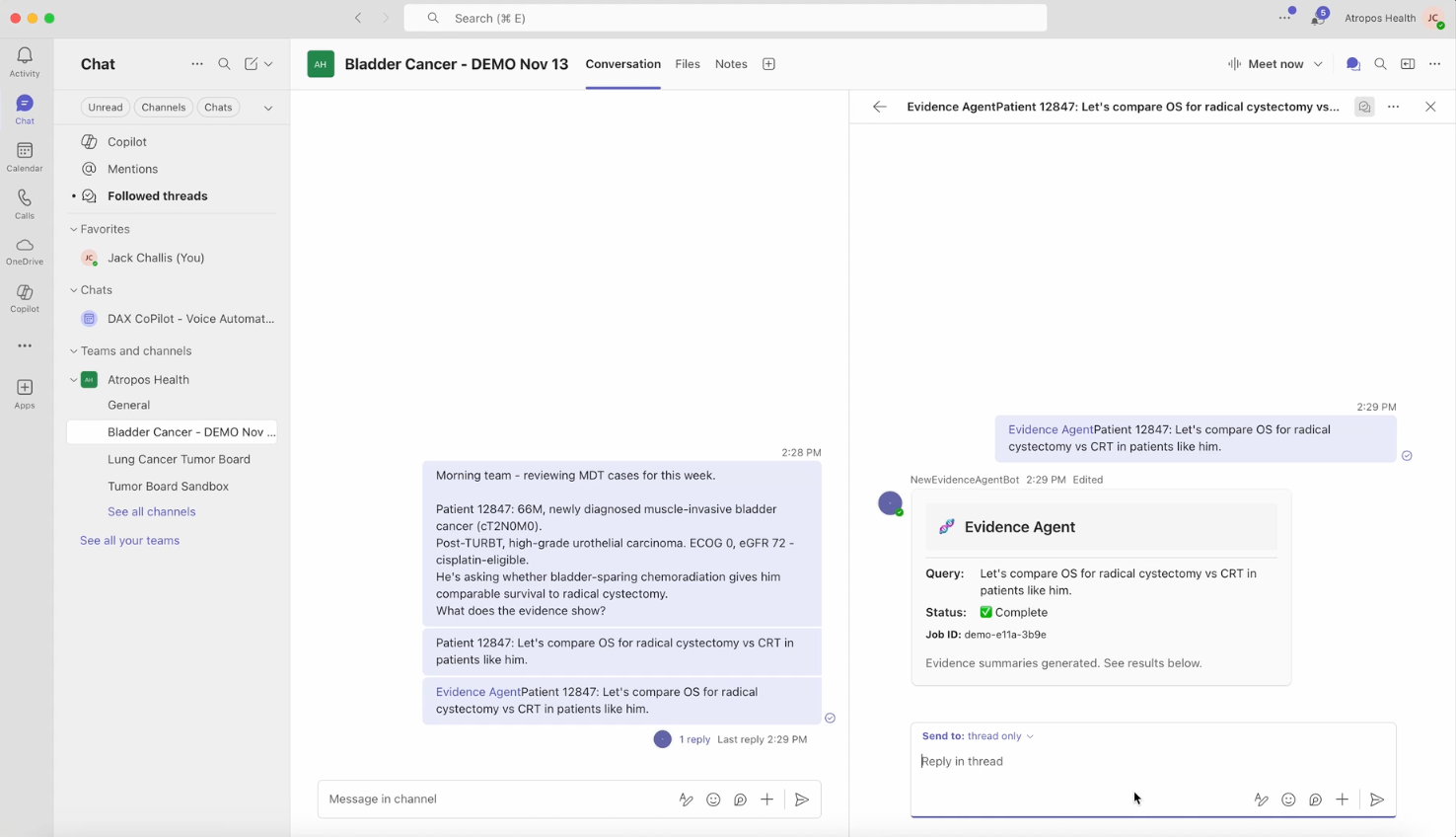
Doctors Can Now Search RWE on Microsoft Teams: Atropos Health Integrates Evidence™ Agent with Microsoft Teams
What You Should Know:
- Atropos Health, a pioneer in translating clinical data into personalized Real-World Evidence (RWE) and insights, has launched the Atropos Evidence™ Agent.
- The expert agent is designed to integrate directly with Microsoft Teams and Microsoft 365 to revolutionize how personalized evidence is delivered to the point of need.
- The integration allows multidisciplinary care team members, such as those participating in Tumor Boards or care coordination
Read More
Folia Health Secures $10.5M Series A to Scale Patient-Driven RWE Platform
What You Should Know:
- Folia Health, the patient-driven health company and pioneer of home-reported outcomes (HROs), committed to transforming lived experiences into structured data insights to advance research and personalized care, today announced the close of a $10.5 million in Series A funding round led by S3 Ventures, alongside longtime partner Crosslink Capital and specialist fund Create Health Ventures.
- The investment will enable the company to double down on its mission to put
Read More
The Next Phase of RWE: Why Integration Matters More Than Data Collection
Real-world evidence (RWE) has become central to evaluating therapies, medical products, and health interventions—providing insight into how they perform outside controlled clinical trials. As healthcare systems seek more timely, representative, and cost-effective approaches to evidence generation, real-world data (RWD) is increasingly used to inform regulatory decisions, guide clinical practice, and shape health policy.
As RWE gains traction, fundamental questions remain: How do we ensure
Read More
Datavant & Boehringer Ingelheim Expand RWE Partnership for 75+ Clinical Trials
What You Should Know:
- Datavant, a leading health data platform, has announced an expanded collaboration with Boehringer Ingelheim to strengthen the pharmaceutical company’s real-world evidence (RWE) capabilities.
- This partnership aims to advance clinical development and commercial strategy across 75 additional clinical trials and multiple new molecular entities (NMEs), leveraging Datavant’s cutting-edge privacy-preserving tokenization and data connectivity technologies.
Datavant
Read More
Advancing Drug Development and Regulatory Compliance with AI-Enhanced Real-World Evidence
In recent years, regulatory bodies, such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have increasingly emphasized the use of real-world data (RWD) in clinical research and regulatory decisions, recognizing its potential to enhance drug development and improve patient outcomes. This shift includes a focus on sourcing RWD that adheres to strict regulatory standards for effective use in drug approvals and postmarket surveillance.
Unlike traditional
Read More
Guardant Health and ConcertAI Partner to Unlock Cancer Insights with Multi-Modal Real-World Data
What You Should Know:
- Guardant Health, a provider in precision oncology, and ConcertAI, a real-world evidence (RWE) and AI technology company in oncology, announced a strategic collaboration that will provide biopharmaceutical companies with access to a first-of-its-kind multi-modal real-world data (RWD) solution.
- The partnership integrates comprehensive patient electronic medical record (EMR) data with genomic and epigenomic tumor profiling information, offering a deeper
Read More
Optimizing Clinical Trials with AI and Data-Driven Insights
While most technology- and innovation-oriented industries have followed the general principles of Moore’s Law, which holds that computing power would double yearly, while costs were cut in half, the life sciences industry appears to be spending more money developing fewer drugs.
According to the latest data, the cost to take a drug candidate from the discovery stage, all the way to market launch, hit $2.3 billion in 2022—a $298 million rise from 2021. Meanwhile, there were
Read More