What You Should Know:
- Movano Health, a health technology innovator, has achieved a significant milestone with the FDA 510(k) clearance of the pulse oximeter integrated into its Evie Ring.
- The FDA clearance paves the way for Movano to pursue new opportunities in health monitoring solutions, including clinical trials, post-clinical management, and remote patient monitoring.
Addressing the Limitations of Traditional Pulse Oximeters
Traditional pulse oximeters can be
Read More
FDA Clearance
RSNA: Nonin Medical Launches First FDA-Cleared OTC Fingertip Pulse Oximeter for Accurate Readings Across Skin Tones
What You Should Know:
- Nonin Medical, a global leader in noninvasive medical monitoring lauches TruO2 OTC, the first over-the-counter (OTC) fingertip pulse oximeter to receive FDA clearance. This innovative device is designed to provide accurate blood oxygen saturation readings for adults across all skin tones, empowering individuals to make informed healthcare decisions.
- Nonin Medical's founder, Phil Isaacson, was the original inventor of the fingertip pulse oximeter. The
Read More
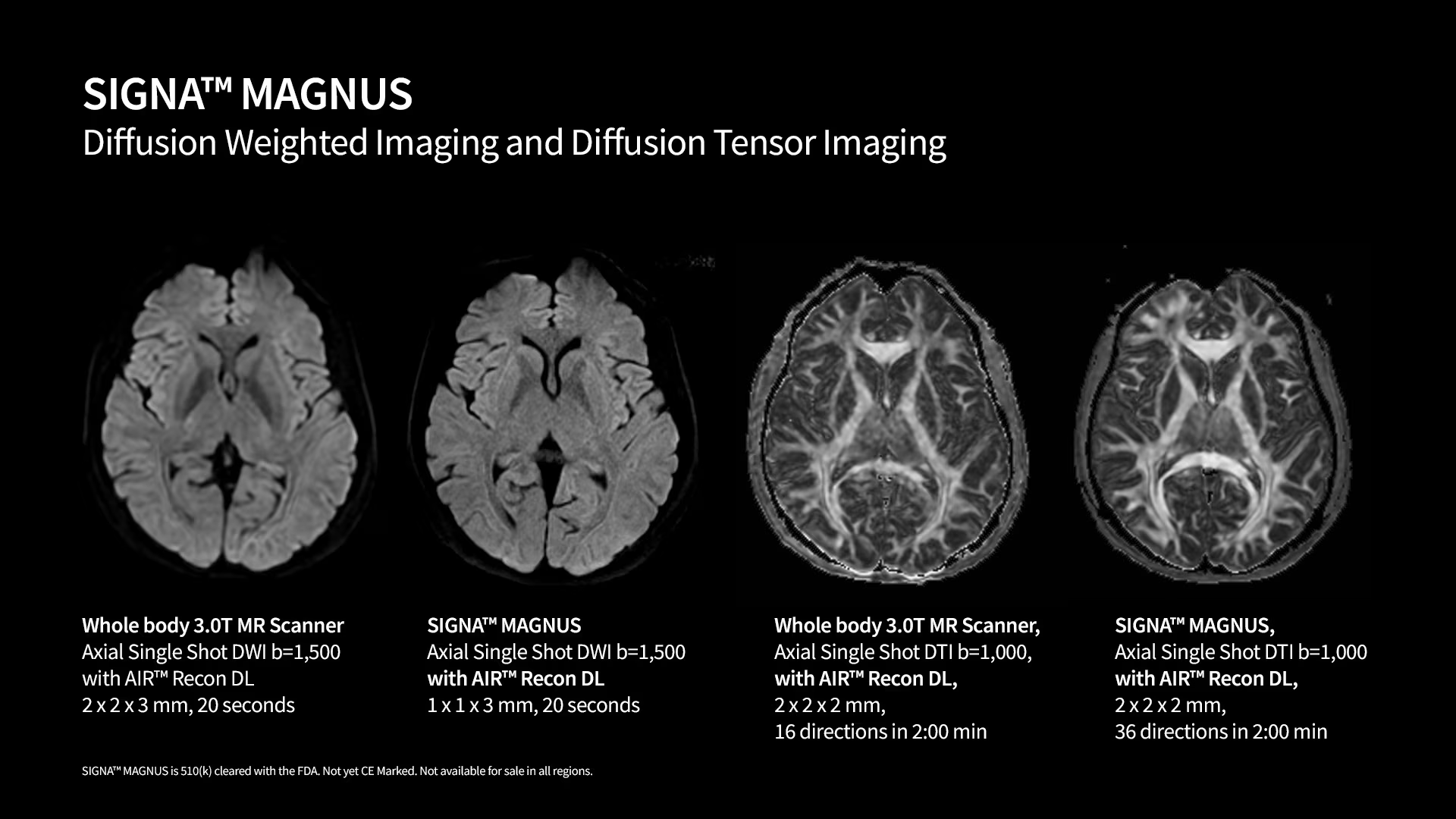
GE HealthCare Receives FDA Clearance for SIGNA™ MAGNUS Head-Only MRI Scanner
What You Should Know:
- GE HealthCare (Nasdaq: GEHC) has received FDA 510(k) clearance for its innovative SIGNA™ MAGNUS, a 3.0T high-performance, head-only magnetic resonance imaging (MRI) scanner.
- This system offers new capabilities for both clinical imaging and neuroscience with the potential to aid in the detection of neurological, oncological, and psychiatric conditions.
SIGNA MAGNUS: Advancing Neuroimaging with Cutting-Edge MRI Technology
The FDA clearance of the SIGNA MAGNUS
Read More
AccurKardia’s AI-Powered ECG Software Earns FDA Breakthrough Device Designation for Aortic Stenosis Detection
What You Should Know:
- AccurKardia, an innovator in ECG-based diagnostics technology, has announced that its Aortic Valve Stenosis (AVS) ECG-based AI screening software has been granted Breakthrough Device Designation by the U.S. Food and Drug Administration (FDA).
- The company’s AVS screening software aims to leverage the ubiquity of the electrocardiogram (ECG) to identify potential cases of AVS within millions of ECGs already present in healthcare system electronic health records in
Read More
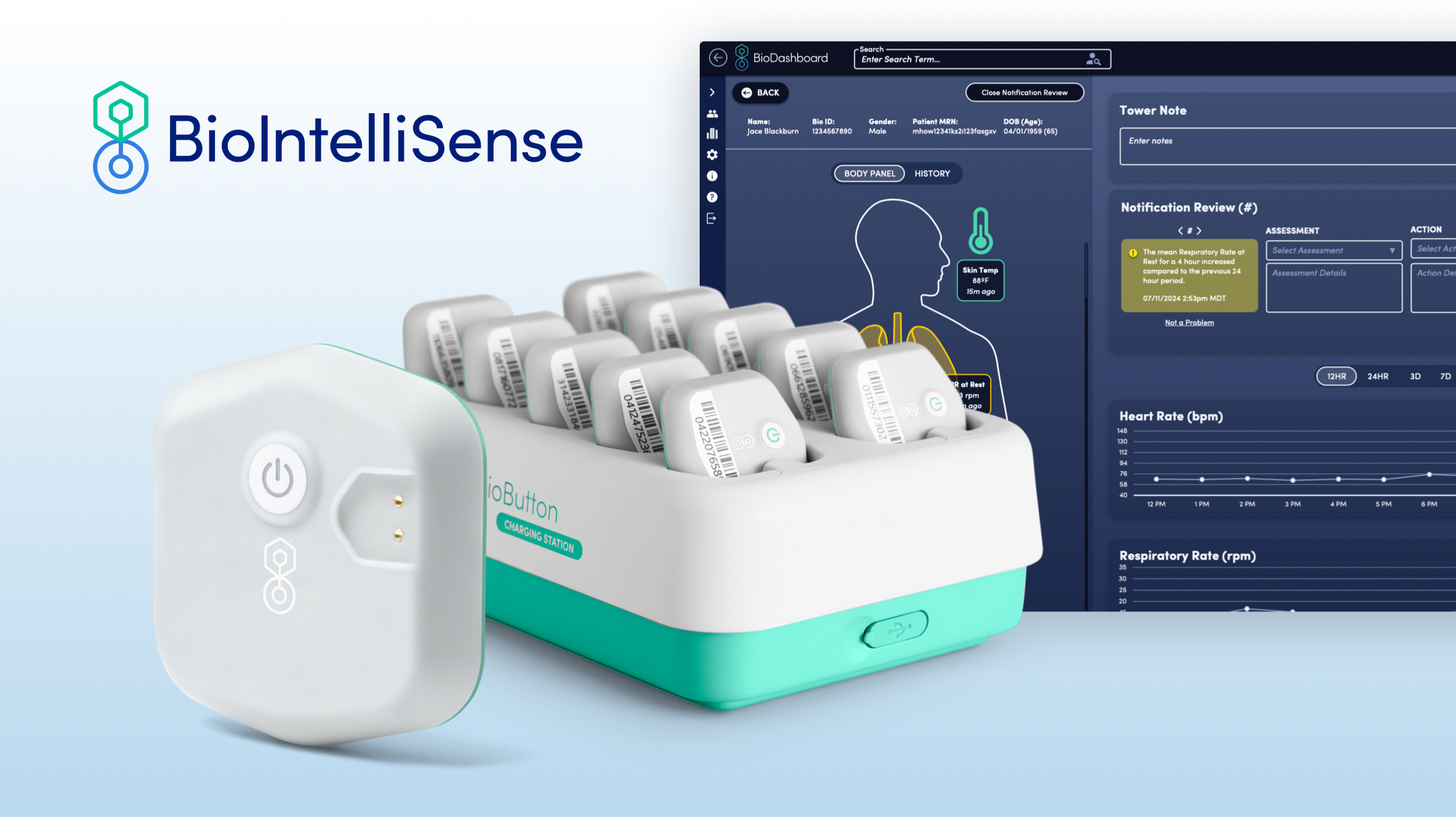
BioIntelliSense Optimizes Hospital Virtual Care with FDA-Cleared Rechargeable BioButton
What You Should Know:
- BioIntelliSense, a leader in continuous health monitoring, has received FDA clearance for its rechargeable BioButton Multi-Patient wearable and BioDashboard system.
- The BioButton is poised to transform hospital virtual care programs by providing a cost-effective and scalable solution for continuous patient monitoring.
Rechargeable and Reusable Wearable
The BioButton Multi-Patient wearable is a game-changer for inpatient settings. This
Read More
GE HealthCare Receives FDA Clearance for Innovative Alzheimer’s Imaging Tool
What You Should Know:
- GE HealthCare announced today that its MIM Software has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for a groundbreaking tool in the fight against Alzheimer's disease.
- The advancement allows MIMneuro, a vendor-neutral software solution, to perform "Centiloid scaling" for analyzing and quantifying amyloid plaque density in the brain using positron emission tomography (PET) imaging.
Impact of Alzheimer's
Read More
Withings Sleep Rx Receives FDA Clearance for Home Sleep Apnea Testing
What You Should Know:
- Withings, a global leader in connected health, has announced that its Sleep Rx sleep apnea device has received clearance from the U.S. Food and Drug Administration (FDA).
- Sleep Rx represents a significant advancement in the diagnosis of sleep apnea, offering a more convenient and accurate alternative to traditional in-lab testing.
Addressing the Underdiagnosis of Sleep Apnea
Sleep apnea is a serious condition that affects millions of people
Read More
Qure.ai Receives FDA Clearance for AI-Powered Lung Nodule Analysis Tool
What You Should Know:
- Qure.ai, a global innovator in medical imaging AI, has today announced a pivotal 510(k) FDA clearance for its AI-powered chest CT solution – qCT LN Quant.
- The new AI solution is now available to support radiologists and pulmonologists in analyzing lung nodules on non-contrast chest CT scans and tracking volumetric growth as part of progression monitoring.
Qure.ai Enhances Lung Cancer Care with AI-Powered qCT LN Quant
Qure.ai has integrated qCT LN Quant into
Read More
AISAP’s AI-Powered Cardiac Ultrasound Receives FDA Clearance
What You Should Know:
- AISAP, a medical technology company specializing in AI-powered point-of-care assisted diagnosis (POCAD) solutions, announced today that it has received FDA 510(k) clearance for its groundbreaking CARDIO software platform.
- AISAP CARDIO is a cloud-based software package that combines four computer-assisted diagnosis (CADx) modules for valvular pathologies and eight key measurements into a single, user-friendly platform.
Point-of-Care
Read More
Diality Receives FDA Clearance for Innovative Hemodialysis System
What You Should Know:
- Diality, a medical device company focused on advancing kidney care, announced today that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Moda-flx Hemodialysis System™.
- The Moda-flx Hemodialysis System is designed to enhance the dialysis experience for both patients and healthcare providers. With its variable flow rate ranges, integrated reverse osmosis water filtration, and user-friendly interface, clinicians
Read More