What You Should Know:
- Verantos, the global leader in high-validity real-world evidence at scale, has launched its Alzheimer’s Disease Pragmatic Registry.
- This registry offers life sciences organizations rich and reliable data on patients with Alzheimer’s disease and related conditions.
Verantos: Leading High-Validity Real-World Evidence
Verantos is the global leader in high-validity real-world evidence at scale. By applying artificial intelligence to comprehensive patient
Read More
Life Sciences | News, Analysis, Insights - HIT Consultant
Tasso & InnoVero to Supply Anti-Doping Test to 2024 Paris Olympics, Expanded Partnership
What You Should Know:
- InnoVero, a global leader in providing innovative, athlete-centric collection technologies to anti-doping organizations worldwide and Tasso, Inc., the leading provider of patient-centric, clinical grade blood collection solutions, today announced the extension of their partnership that was first initiated in 2021.
- In addition to collaboratively developing new athlete-centric solutions, InnoVero will continue to serve as the exclusive worldwide distributor of Tasso
Read More
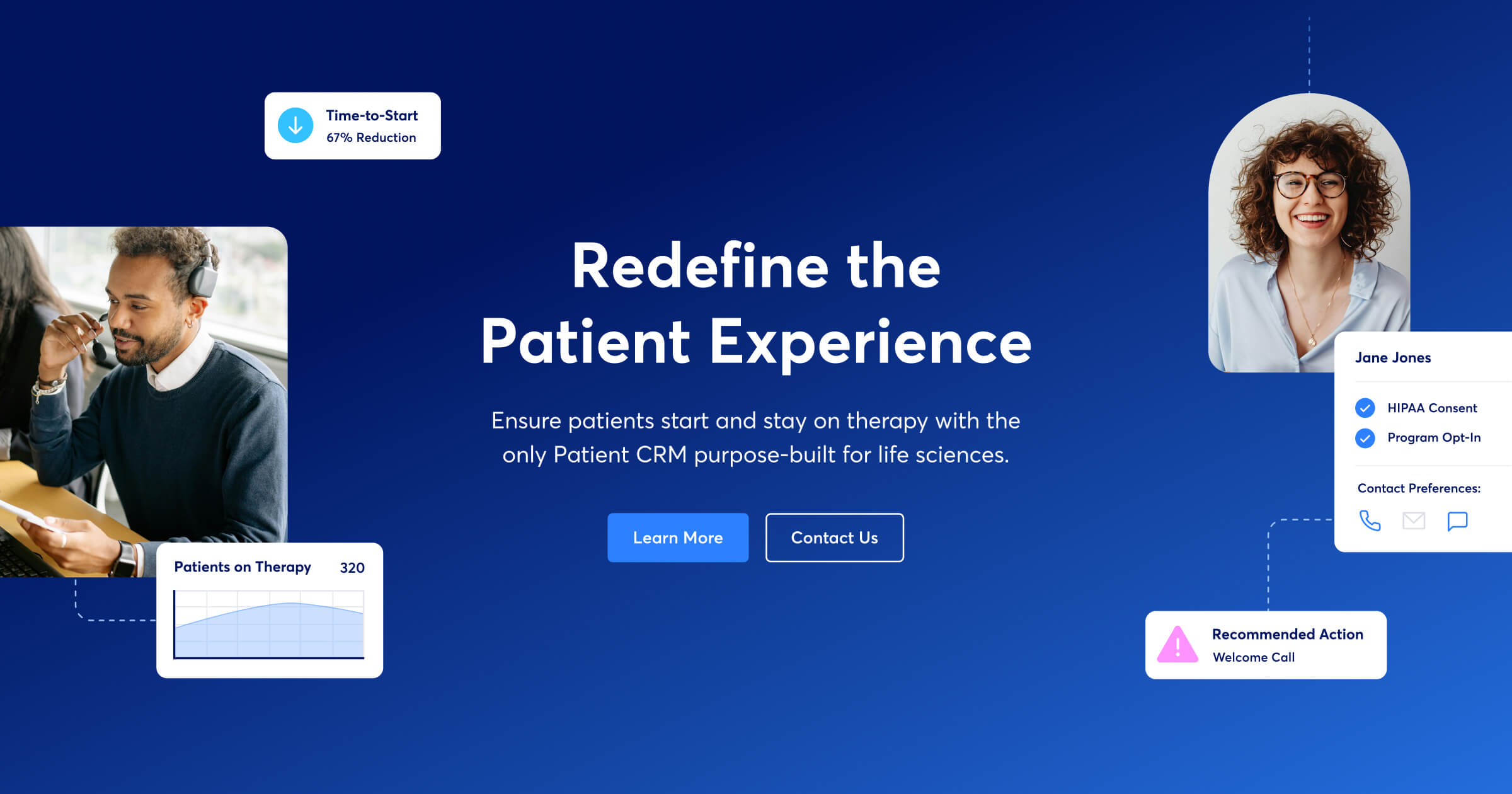
Courier Health Raises $16.5M for First Patient CRM for Biopharma
What You Should Know:
- Courier Health, a technology company transforming patient experiences in the life sciences industry raises $16.5M in Series A funding led by Norwest Venture Partners with participation from existing investor Work-Bench.
- The investment fuels Courier Health's mission to provide biopharma companies with the tools to manage and personalize the patient journey for life-altering therapies.
Addressing the Rise of Specialty Medicines
The landscape of
Read More
Pangea Biomed’s ENLIGHT-DP Predicts Cancer Treatment Response from Simple Scans
What You Should Know:
- Pangea Biomed, a leader in precision oncology, announced a significant breakthrough in its ENLIGHT cancer response predictor. A new study published in Nature Cancer demonstrates the effectiveness of their ENLIGHT-DP platform, revealing its ability to predict cancer treatment response across various types and drugs using only routine pathology slides.
- While these findings are promising, further validation and testing are planned for regulatory
Read More
Biopharma at a Crossroads: AI Offers Key to R&D Reinvention
What You Should Know:
- A new report by Accenture, "Reinventing R&D in the Age of AI," paints a clear picture: the biopharmaceutical industry stands at a critical juncture. Companies that embrace intelligent technologies like AI and machine learning are poised to become the future's leaders, while those clinging to outdated methods risk falling behind.
- Accenture's report serves as a roadmap, offering valuable insights and practical steps for companies ready to embrace the
Read More
VenoStent Raises $20M for Dialysis Solution, Secures $3.6M NIH Grant for U.S. Dialysis Therapeutic Clinical Trial
What You Should Know:
- VenoStent, Inc., a medical device company pioneering a new approach to improve dialysis patient outcomes, announced the successful completion of its Series A funding round. The round closed at $20M, with Norwest Venture Partners contributing an additional $4M to join Good Growth Capital and IAG Capital Partners as co-leads.
- VenoStent also welcomes Dr. Zack Scott, Norwest General Partner, and Dr. Ehi Akhirome, Norwest Investor, to its board of
Read More
Clinical Trial Diversity: Pharma Takes Action to Address Disparities
What You Should Know:
- Trinity Life Sciences, a leader in global life sciences commercialization solutions, has released a new white paper titled "Diversity in Clinical Trials: Life Sciences Initiatives and Challenges in Light of the FDA's Latest Guidance."
- The paper sheds light on the critical issue of racial and ethnic diversity in clinical trials, exploring current industry initiatives, their effectiveness, and the future landscape shaped by the FDA's evolving
Read More
How Vertical Commercial Optimization Platforms Help Pharma Companies Do Omnichannel Marketing Right
In a world full of options, companies need to compete on every prospect and existing customer. As the number of potential channels to reach customers increases, companies need to build strategies for interacting with the right customers, through the right channels, at the right time and with the right messages. Omnichannel marketing has emerged as an effective and efficient means for optimizing this customer engagement. However, while omnichannel is far from being simple in any industry, it
Read More
Proscia and Nucleai Partner to Integrate Predictive Biomarker Solutions
What You Should Know:
- Proscia, a leader in artificial intelligence (AI)-powered pathology solutions, and Nucleai, a leading spatial AI biomarker company, announced a strategic partnership today. This collaboration aims to revolutionize precision medicine by integrating Nucleai's advanced biomarker technology with Proscia's Concentriq® software platform.
- By harnessing the power of AI and digital pathology, this partnership offers the potential to fundamentally improve the
Read More
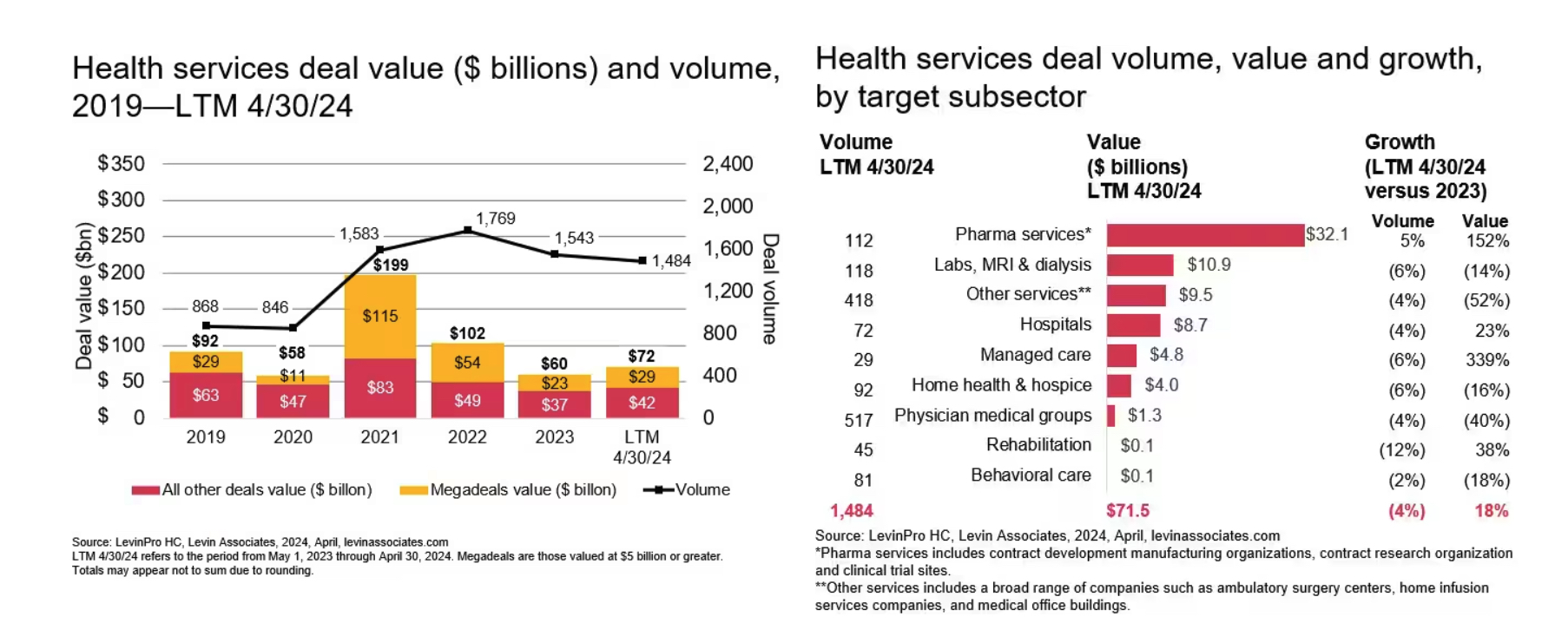
PwC: Pharma & Biotech Dealmaking Heats Up Despite Uncertainties
What You Should Know:
- The pharmaceutical and life sciences (PLS) sector is experiencing a resurgence in dealmaking activity, with a 20% increase in deal volume over the past year reported, according to PwC’s latest US Midyear Deals 2024 Outlook for Pharmaceutical and Life Sciences.
- M&A activity in the PLS sector is expected to remain strong throughout 2024 and potentially into 2025. Clarity on factors like US election outcomes and Federal Reserve interest rate decisions
Read More