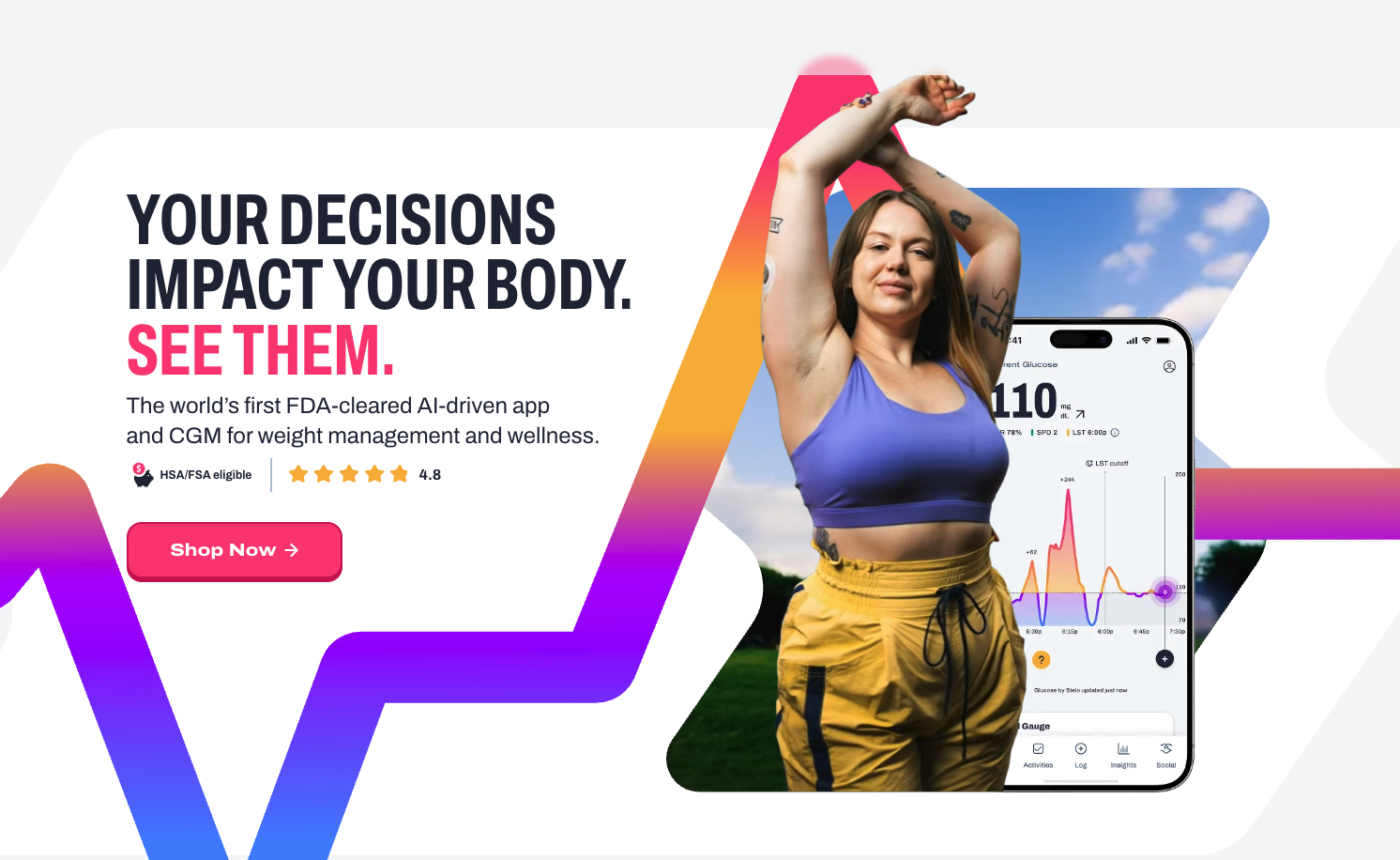
What You Should Know:
– Signos, a metabolic and weight management company, has announced the launch of the Signos Glucose Monitoring System.
– The system is the first FDA-cleared, over-the-counter glucose monitoring system for weight management. It is also the first non-pharmacological approach cleared by the FDA for glucose management that not only helps those who are overweight but also helps prevent those who are at-risk.
Enterprise-Grade Capabilities, Consumer-Friendly Experience
In the U.S., nearly 74% of Americans are overweight or obese, and 88% are metabolically unhealthy, which fuels conditions like type II diabetes, prediabetes, and hypertension. The Signos system is designed to address this by using real-time data to show how a user’s lifestyle impacts their health. It then guides them to make small, sustainable changes.
The Signos system integrates hundreds of millions of data points with each user’s unique profile and is paired with Stelo by Dexcom and Signos’ proprietary AI. The system shows how food, activity, stress, and sleep affect the body in real-time. This combination of precision health and a user-friendly experience is designed for easy adoption, measurable outcomes, and scalable impact.
The system offers several key benefits for different stakeholders:
- For Employers and Health Plans: They can monitor adoption, adherence, and outcomes instantly, which eliminates guesswork and waste. The solution works alongside GLP-1s or other programs and is a turnkey deployment that requires no integrations and can be rolled out in weeks.
- For Insurance Carriers: They can lower chronic disease costs with proactive, preventative tools that are supported by real-time population-level data.
- For Clinical Partners: They can access real-time patient data to reinforce healthy decisions between visits.
Availability
The Signos Glucose Monitoring System is available now. For enterprise and partnership inquiries, visit signos.com/business.
