What You Should Know:
– A new era in the treatment of chronic venous insufficiency (CVI) began on June 11th, 2024, with the successful implantation of the Philips Duo Venous Stent System outside of a clinical trial setting.
– Dr. Erin Murphy, a leading vascular surgeon at Atrium Health’s Sanger Heart & Vascular Institute, performed the procedure, marking a significant milestone for patients suffering from this debilitating condition.
CVI: A Common Yet Complex Cardiovascular Issue
CVI, affecting an estimated 25 million people worldwide, arises from venous outflow obstruction, often caused by blood clots [1, 2]. Ranked as the third most common cardiovascular disease [2], CVI presents unique challenges due to the complexities of venous anatomy and obstructions.
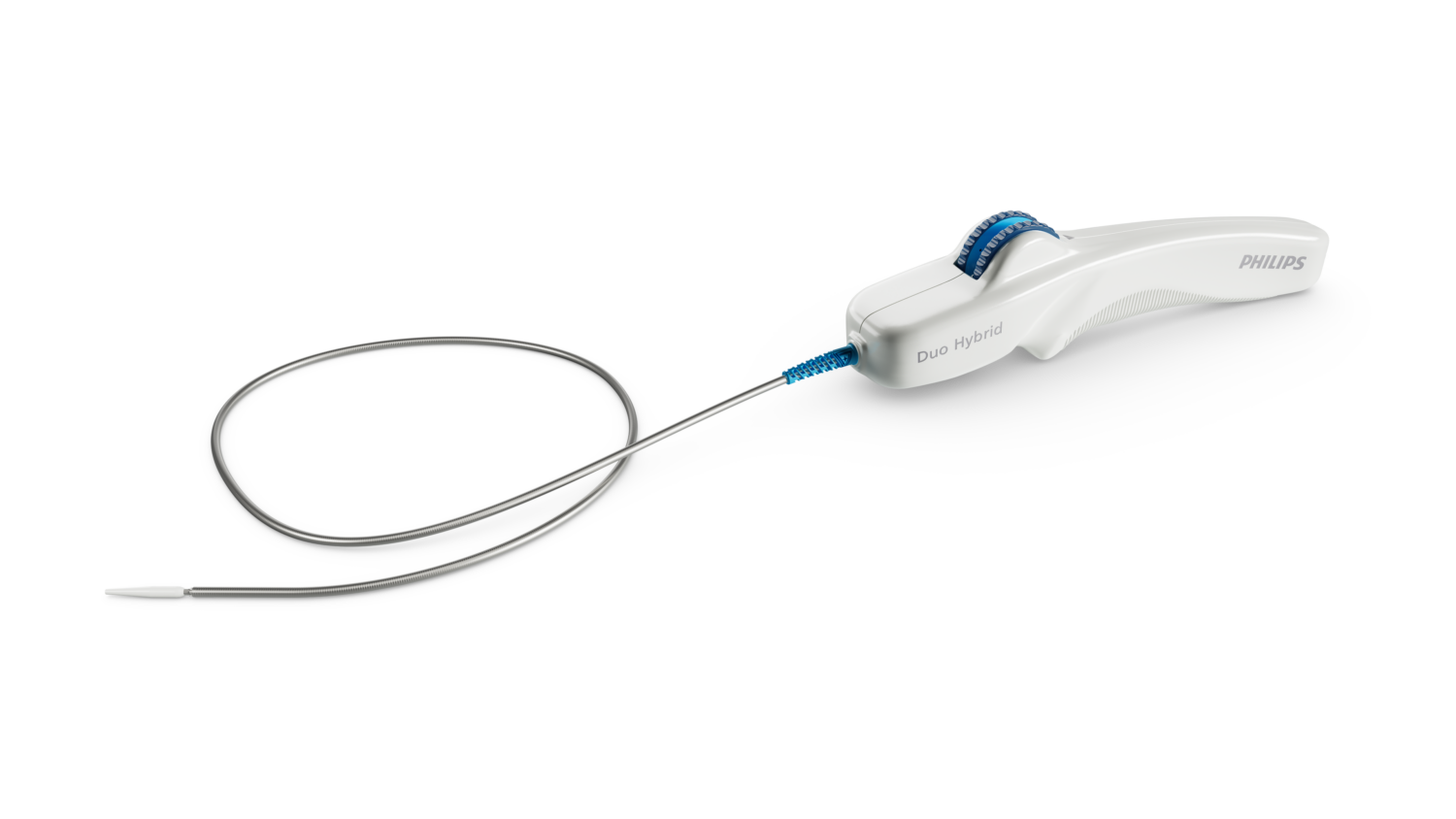
The Duo Venous Stent System: A Tailored Solution
The Philips Duo Venous Stent System offers a novel approach to addressing CVI. This FDA-approved device comprises two stents – the Duo Hybrid and Duo Extend – designed for various sizes and venous configurations. The Duo Hybrid’s unique design integrates multiple zones with varying mechanical properties within a single stent [3]. For longer lesions, the Duo Extend seamlessly connects to the Duo Hybrid, extending treatment coverage. This innovative design aims to minimize the risk of stent fracture and corrosion, while enabling stent placement in smaller caudal veins [3].
Clinical Trial Success Paves the Way for Real-World Application
The VIVID clinical trial played a critical role in bringing the Duo Venous Stent System to market. This global, multi-center study evaluated the device’s safety and efficacy in treating nonmalignant iliofemoral occlusive disease. The VIVID trial enrolled over 160 patients across three categories: non-thrombotic iliac vein lesions (NIVL), post-thrombotic syndrome (PTS), and acute deep vein thrombosis (aDVT). The results were highly promising, exceeding pre-established performance goals for both safety and efficacy at the 12-month mark. The primary patency rate reached an impressive 90.2%, surpassing the target of 77.3%, while the primary safety endpoint achieved a remarkable 98.7%, exceeding the goal of 89%.
Beyond Efficacy: Improved Quality of Life
The VIVID study also explored the impact of the Duo Venous Stent System on patients’ quality of life. Assessments performed at baseline and 12 months post-implantation revealed sustained improvements in areas such as clinical presentation, venous function, and overall well-being. This comprehensive approach underscores the potential of the Duo Venous Stent System to significantly enhance patients’ lives beyond simply addressing the underlying vascular condition.
A New Era of Venous Care
The successful implantation of the Duo Venous Stent System by Dr. Murphy signifies a new chapter in CVI treatment. This FDA-approved device, backed by robust clinical trial data, offers a promising solution for patients seeking relief from the challenges associated with CVI. With its innovative design and focus on both efficacy and improved quality of life, the Duo Venous Stent System has the potential to transform the landscape of venous care.
“Duo is the first stent that offers a differential design for the challenges of venous anatomy – a focal area that withstands the forces of compression as well as the flexibility to accommodate curvature of the vessel,” said Dr Kush Desai, a highly regarded Interventional radiologist and associate professor of Radiology, Surgery and Medicine at Northwestern University in Chicago.
