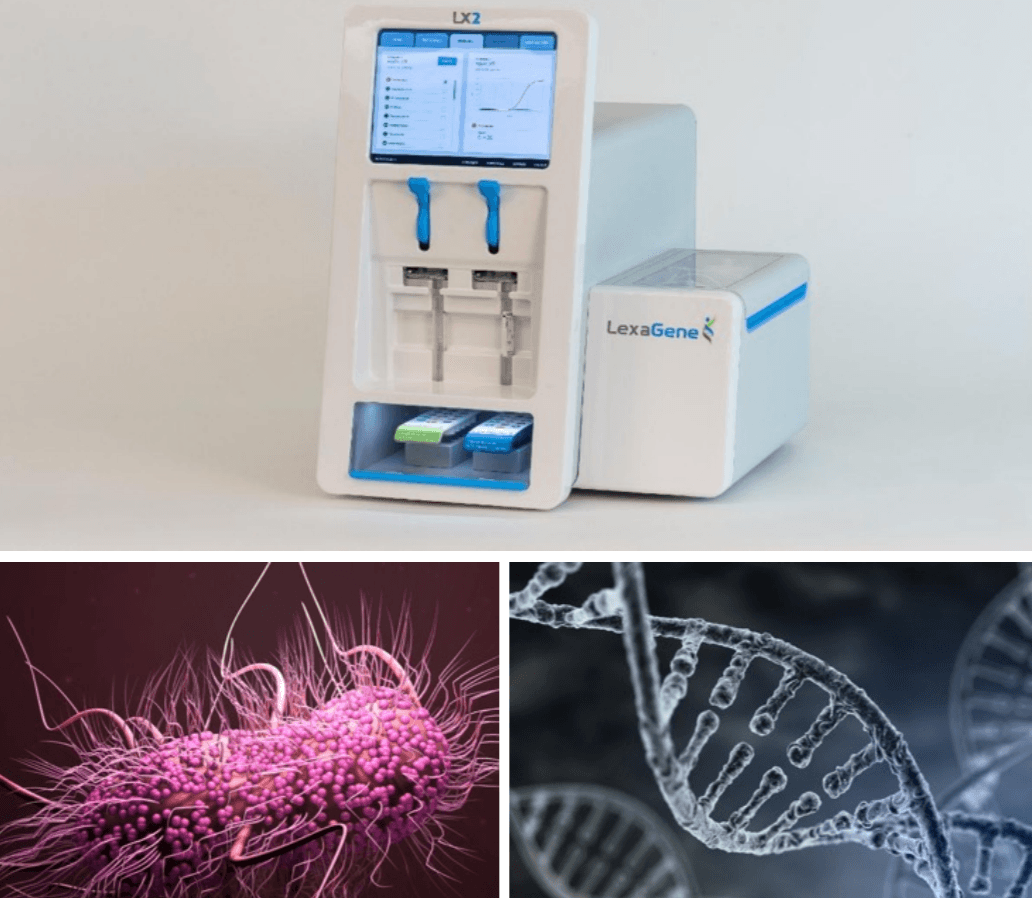
– LexaGene has created a genetic analyzer that can identify specific bacteria and viruses as well as any antibiotic/antimicrobial resistance that they have, within just one hour.
– The device does this completely automatically, without the need for trained lab technicians, by amplifying the genetic material in the sample to identify it, rather than waiting for a culture which can take several days.
LexaGene Holdings, Inc., a biotechnology company that develops genetic analyzers for
the rapid detection of pathogens and other molecular markers, has received many
inquiries regarding the ability of LexaGene’s technology for detecting
coronavirus.
This new pathogen first emerged in mid-December
when several workers in the Huanan Seafood Market of Wuhan, China displayed
symptoms of severe pneumonia from an unknown pathogen. On January 9th, China
CDC reported that the infections were due to a novel coronavirus, currently
referred to as 2019-nCoV. In an attempt to control disease spread, the
Chinese government has quarantined entire cities, and international airports are
now screening travelers from affected areas.
Despite these efforts, the World Health Organization reported that by January 26th, over 2,000 cases had been confirmed and 56 deaths were reported.1 Human-to-human transmission has been confirmed and the disease has quickly spread to ten other countries, including the United States, France, Canada, and Australia. The risk of a pandemic is now very real.
Dr. Regan continues, “Developing a new diagnostic test to detect this coronavirus is important and has already been done by numerous groups.2 The challenge is bringing these manually performed tests out of the specialized reference laboratories that can take days to return results and instead to the points-of-need, which are the clinics, hospitals, and airports where answers are needed in one hour or less. Currently, no technology exists that allows for these locations to readily start screening for a new threat within days of an outbreak being identified. LexaGene is addressing this urgent problem and has developed the first-ever, easy-to-use, open-access diagnostic analyzer that is designed to help control the spread of deadly outbreaks such as this 2019 coronavirus.”
LexaGene’s microfluidic technology is designed for on-site use and screens for both common pathogens and new bio-threats. This is possible due to its open-access feature that permits non-technical operators to quickly add new tests to detect novel pathogens.
Availability/Timeline
LexaGene is now finalizing the design of its commercial instrument, which it anticipates starting to manufacture in a few months, with the expectation of achieving first commercial sales in Q3 of this year. For implementation in hospitals, clinics, airports, and other locations interested in processing human samples for clinical purposes, the Company will need either Emergency Use Authorization (EUA) or 510(k) clearance from the Food and Drug Administration (FDA).
Why It Matters
“LexaGene’s technology is ideally suited to identify novel pathogens such as coronavirus. Our genetic analyzer can quickly detect new pathogens in just one hour, on-site. Currently, the traditional process requires that samples from sick individuals must be transported to laboratories for manual processing. This is extremely inefficient and introduces a significant time-delay that can have severe consequences for disease spread. Today’s coronavirus outbreak highlights why LexaGene’s technology is needed so desperately,” said Dr. Jack Regan, LexaGene’s CEO and Founder.
