What You Should Know:
– Ceribell’s LVO Stroke Breakthrough In January 2026, the FDA granted Breakthrough Device Designation to Ceribell for its LVO (Large Vessel Occlusion) stroke monitor.
– This first-in-class tool applies an AI algorithm to portable EEG hardware to detect in-hospital strokes—which account for 17% of all strokes and have a mortality rate 3x higher than community-onset strokes. By providing continuous bedside monitoring, it aims to eliminate the “detection gap” in sedated or post-surgical patients.
The “Detection Gap”: Why In-Hospital Strokes Are Deadlier
In-hospital-onset strokes (IHOS) are a clinical blind spot. Unlike a patient arriving at the ER with obvious symptoms, IHOS patients are often:
- Recovering from surgery or heavily sedated.
- Intubated or ventilated, making verbal neurological checks impossible.
- Located in non-neurological units where staff are not trained to spot subtle “brain failure” signs.
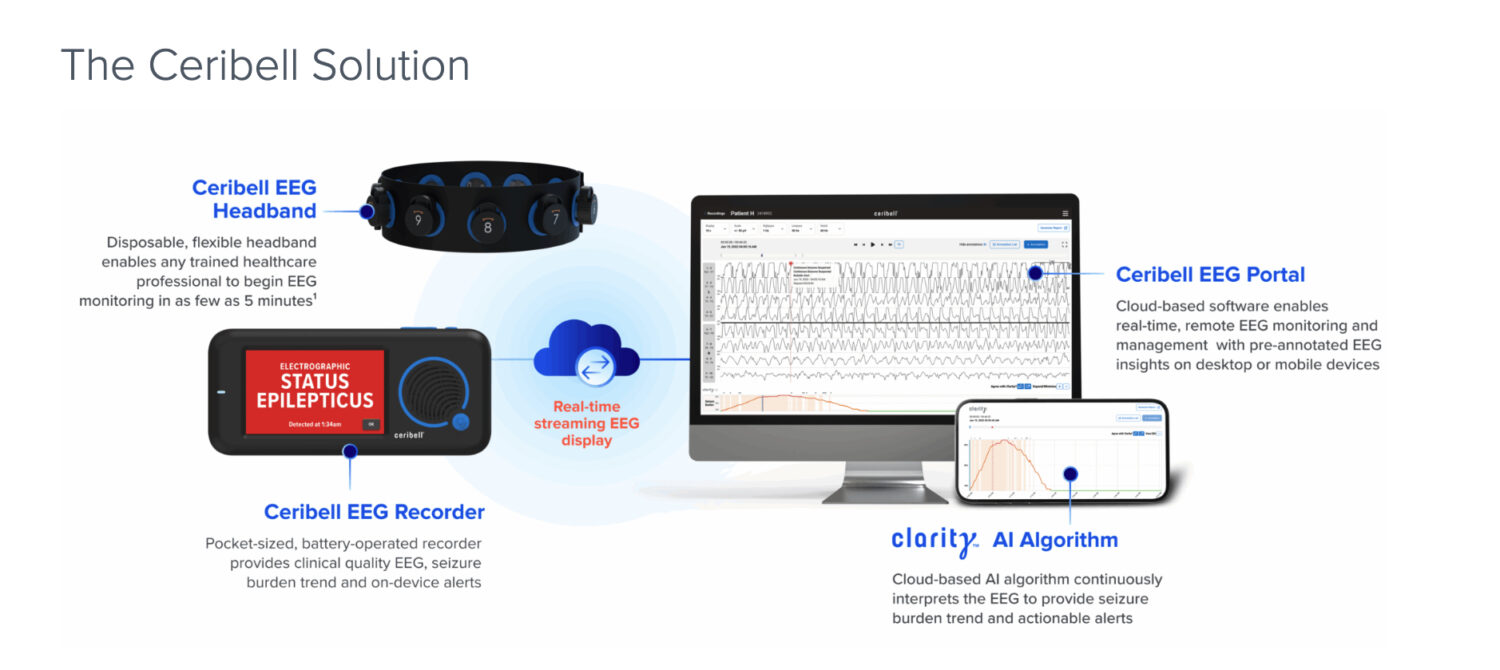
Technical Architecture: Leverage of the ‘Vital Sign’ Platform
Ceribell’s strategy is a masterclass in infrastructure reuse. Instead of requiring new hardware, the LVO monitor uses the existing Ceribell point-of-care EEG system (the 10-electrode headband) and applies a new AI-based algorithm.
- Continuous Monitoring: Unlike a “one-and-done” CT scan, the EEG monitor can remain on the patient, alerting teams the moment brain electrical patterns shift.
- Clinical Synergy: This builds on Ceribell’s recent FDA 510(k) clearances for the Clarity® algorithm in neonates (November 2025) and its delirium monitoring solution (December 2025).
Investors responded favorably, with Ceribell’s stock (CBLL) jumping 8% on the news. However, the “Breakthrough” tag is not an approval. The system must still prove its sensitivity and specificity in the noisy environment of a busy hospital floor.
