What You Should Know:
– Artera, the developer of multimodal artificial intelligence (MMAI)-based prognostic and predictive cancer tests, announced today that the U.S. Food and Drug Administration (FDA) has granted De Novo authorization for ArteraAI Prostate, establishing it as the first and only AI-powered software authorized to prognosticate long-term outcomes for patients with non-metastatic prostate cancer.
– ArteraAI Prostate is now recognized as an FDA-regulated Software as a Medical Device (SaMD).
Artera Secures FDA De Novo Authorization for AI-Powered Prostate Cancer Diagnostic Platform
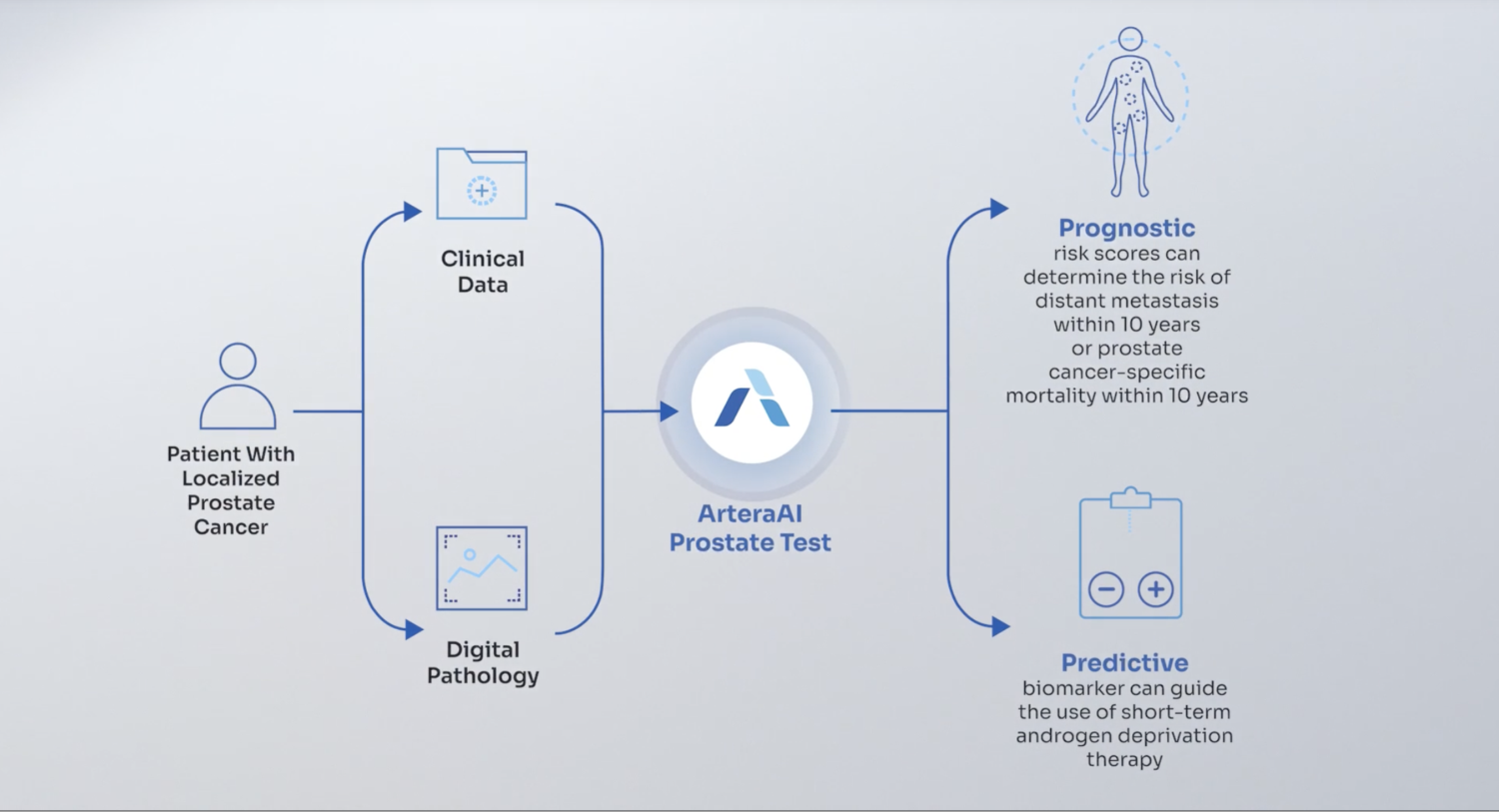
Artera, a global leader in precision oncology, is redefining cancer care through its multimodal artificial intelligence (MMAI) platform, which integrates digitized biopsy images with patient clinical data to assess cancer aggressiveness and predict therapy benefit. Validated across multiple Phase 3 randomized trials and various cancer types, Artera’s MMAI technology is deployed globally through a suite of innovative products.
The company’s flagship ArteraAI Prostate Test—commercially available in the U.S. as a laboratory-developed test and internationally via distribution partners—is the first test to provide both prognostic and predictive insights for prostate cancer, enabling clinicians and patients to make timely, evidence-based treatment decisions.
Regulatory Milestone and Expanded Capabilities
Artera has earned FDA De Novo authorization for its ArteraAI Prostate medical device software, establishing a new product code category for future AI-powered digital pathology risk-stratification tools. This authorization, paired with UKCA certification for the ArteraAI Breast Test, ArteraAI Prostate Biopsy Assay, and ArteraAI Prostate, extends the platform’s reach across key global markets.
- Point-of-Diagnosis Integration – Enables qualified pathology labs in the U.S. to deliver actionable prostate cancer insights at the time of diagnosis, reducing delays in care.
- Predetermined Change Control Plan – Allows platform expansion to additional digital pathology scanners without requiring new 510(k) submissions.
- Breakthrough Device Designation – Preceded the De Novo authorization, underscoring the innovation’s clinical significance.
“This is a defining moment for AI in cancer care,” said Andre Esteva, CEO and co-founder of Artera. “The FDA’s decision validates our vision to deliver AI-guided tools that enable data-backed, tailored treatments for each patient—enhancing confidence throughout the cancer journey and ultimately saving more lives.”
With its latest regulatory clearance, Artera is positioned to scale its MMAI-powered portfolio, accelerating the adoption of precision oncology solutions and advancing the standard of personalized cancer care worldwide.
