What You Should Know:
– Sware, the leading provider of comprehensive GxP validation solutions for innovative life sciences and technology companies, today announced that it will deliver GxP computer systems validation of Salesforce Life Sciences Cloud.
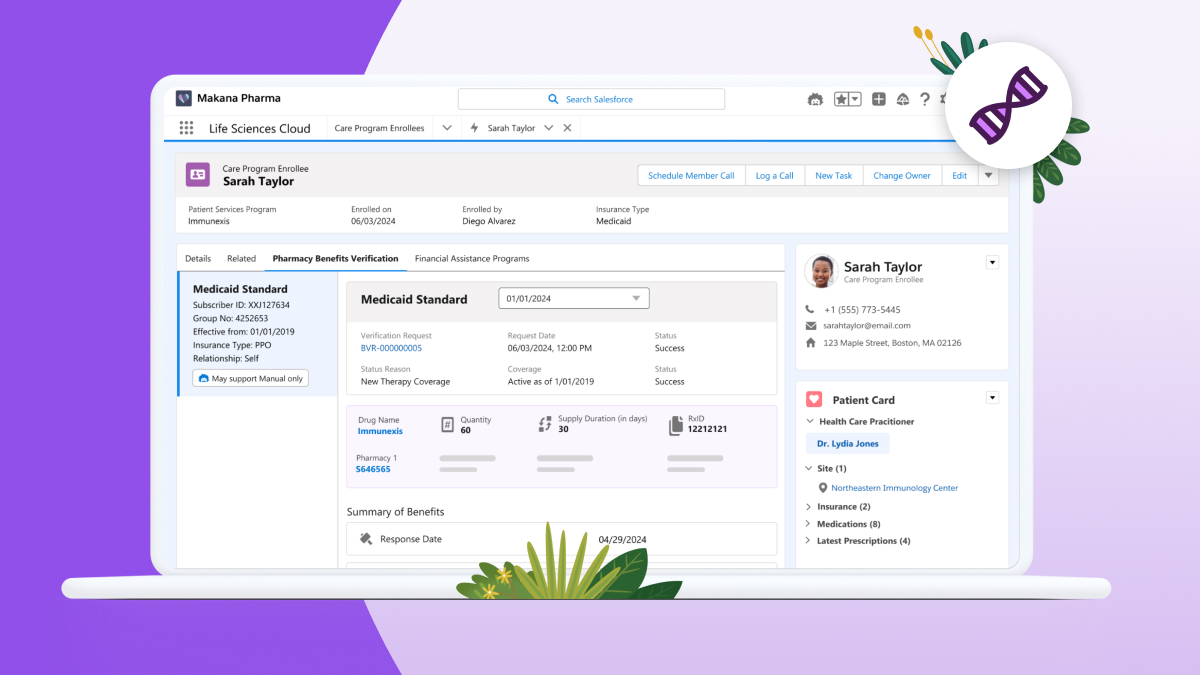
– Life Sciences Cloud empowers pharmaceutical and medical technology organizations to accelerate drug and device development, recruit and retain patients across clinical trials, and leverage AI to deliver personalized customer experiences.
Driving Scalable, Audit-Ready Compliance: Sware’s AI-Powered GxP Validation for Life Sciences Cloud
Sware, a leader in intelligent validation solutions, is redefining GxP compliance for the life sciences industry through advanced automation and AI integration. Its flagship platform, Res_Q, streamlines computer system validation and regulatory readiness, enabling faster digital transformation while ensuring consistent adherence to compliance standards.
Now integrated into Salesforce’s Life Sciences Cloud, Res_Q empowers organizations to achieve modern, efficient, and audit-ready compliance by:
- Automating end-to-end validation, eliminating manual efforts, and accelerating adoption timelines,
- Providing comprehensive audit support with industry-standard certifications and third-party reports,
- Enabling seamless integration with existing compliance tools through a data-driven framework,
- Delivering real-time visibility via intuitive dashboards that track compliance status.
By leveraging Res_Q, Life Sciences Cloud users can minimize regulatory risk while safeguarding patient safety, data integrity, product quality, and information security. The solution enhances operational efficiency and equips quality and regulatory teams with actionable insights, significantly reducing compliance burden across the enterprise.
Bryan Ennis, Founder and Chief Quality Officer at Sware, emphasized the strategic value of the partnership: “Sware is helping Life Sciences Cloud redefine GxP compliance with automation, intelligence, and seamless integration. Our technology empowers organizations to stay ahead of regulatory challenges by delivering a proactive, data-driven approach to quality.”
Trusted by leading pharmaceutical, biotech, and software companies, Sware delivers scalable, future-ready solutions for organizations ranging from emerging biotechs to multinational pharma leaders. As the industry continues its digital and AI evolution, Sware remains committed to making compliance more intelligent, efficient, and resilient.
