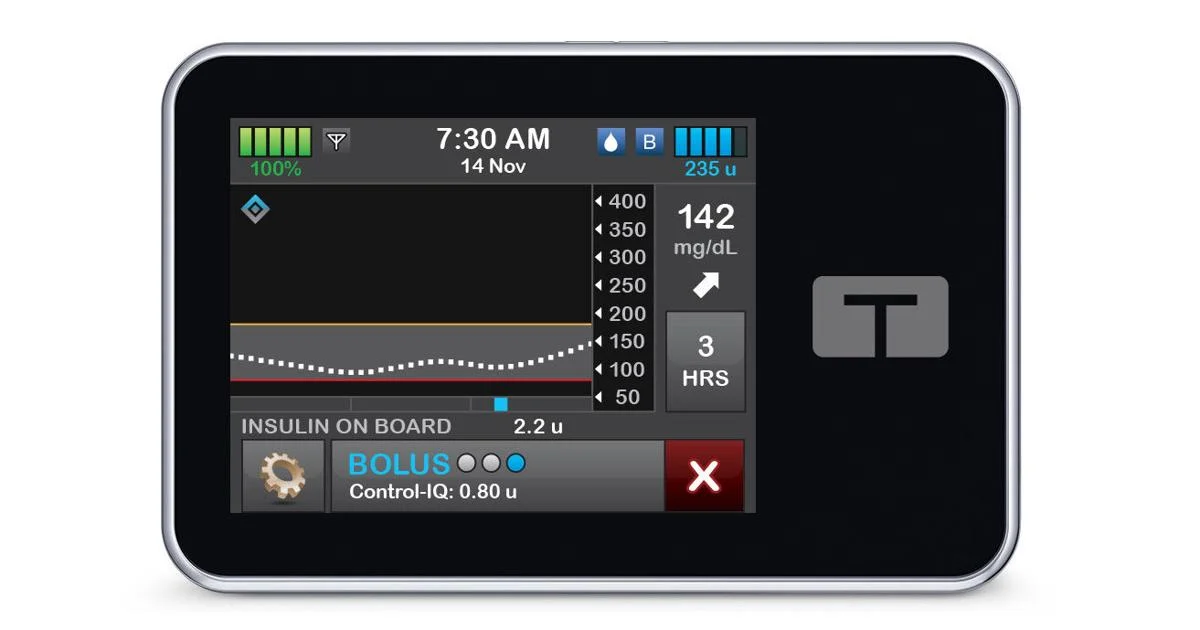
What You Should Know:
– Tandem Diabetes Care, a insulin delivery and diabetes technology company, announced that its Control-IQ+ technology has received clearance from the U.S. Food and Drug Administration (FDA) for use by adults with type 2 diabetes.
– The expanded indication builds on the success of Control-IQ+ in type 1 diabetes and offers a new automated insulin delivery (AID) option for individuals with type 2 diabetes.
Improve Glycemic Control for Adults with Type 2 Diabetes
Control-IQ+ technology is designed to help people with diabetes achieve better glycemic control by automatically adjusting insulin delivery based on continuous glucose monitoring (CGM) data. The system learns an individual’s insulin needs and makes adjustments throughout the day to help keep glucose levels in a healthy range.
This FDA clearance for type 2 diabetes is based on the results of a pivotal trial involving over 300 participants. The trial demonstrated that the t:slim X2 pump with Control-IQ+ technology led to significant improvements in glycemic control compared to traditional multiple daily injections.
Expanding Access to AID
This FDA clearance expands access to advanced AID technology for individuals with type 2 diabetes. Control-IQ+ is expected to be available for new and existing customers in the United States in March 2025.
“Type 2 diabetes affects millions of Americans and increases the risk of serious health conditions, including heart disease, stroke, kidney disease, and nerve damage, reinforcing the importance of consistent management of blood sugar,” said Jordan Pinsker, MD, chief medical officer. “More than 2 million people in the U.S. rely on intensive insulin therapy to manage their type 2 diabetes, and we are proud to bring this life-changing technology to a group that has historically had limited options for diabetes management.”
