What You Should Know:
– Philips, a global leader in health technology, has announced a significant milestone in treating peripheral artery disease (PAD) by enrolling the first patient in the U.S. THOR IDE clinical trial.
– The study is set to evaluate the safety and efficacy of a new catheter developed by Philips, which uniquely combines laser atherectomy and intravascular lithotripsy into a single device. This innovative approach allows physicians to treat complex, calcified arterial lesions in a single procedure, potentially reducing the number of devices and interventions needed to restore blood flow to the limbs.
Pioneering Combined Therapy for PAD
Peripheral artery disease, which restricts blood flow to the limbs, affects millions of people worldwide, especially older adults and those with diabetes or high cholesterol. PAD often leads to severe pain, ulcers, and even limb amputation if untreated. Patients with calcified lesions—a common and difficult-to-treat manifestation of PAD—usually face multiple, complex treatments involving separate devices for atherectomy and intravascular lithotripsy. The Philips device combines these treatments into one procedure, making PAD treatment simpler and potentially less risky.
The Cardiovascular Institute of the South in Louisiana successfully treated the first U.S. patient enrolled in the THOR IDE trial. A 78-year-old male with peripheral vascular disease underwent treatment using the Philips catheter, marking a significant step forward in Philips’ clinical research program. This initial procedure highlights the potential of the device to streamline the patient experience and reduce hospital visits by combining two critical PAD therapies in one device.
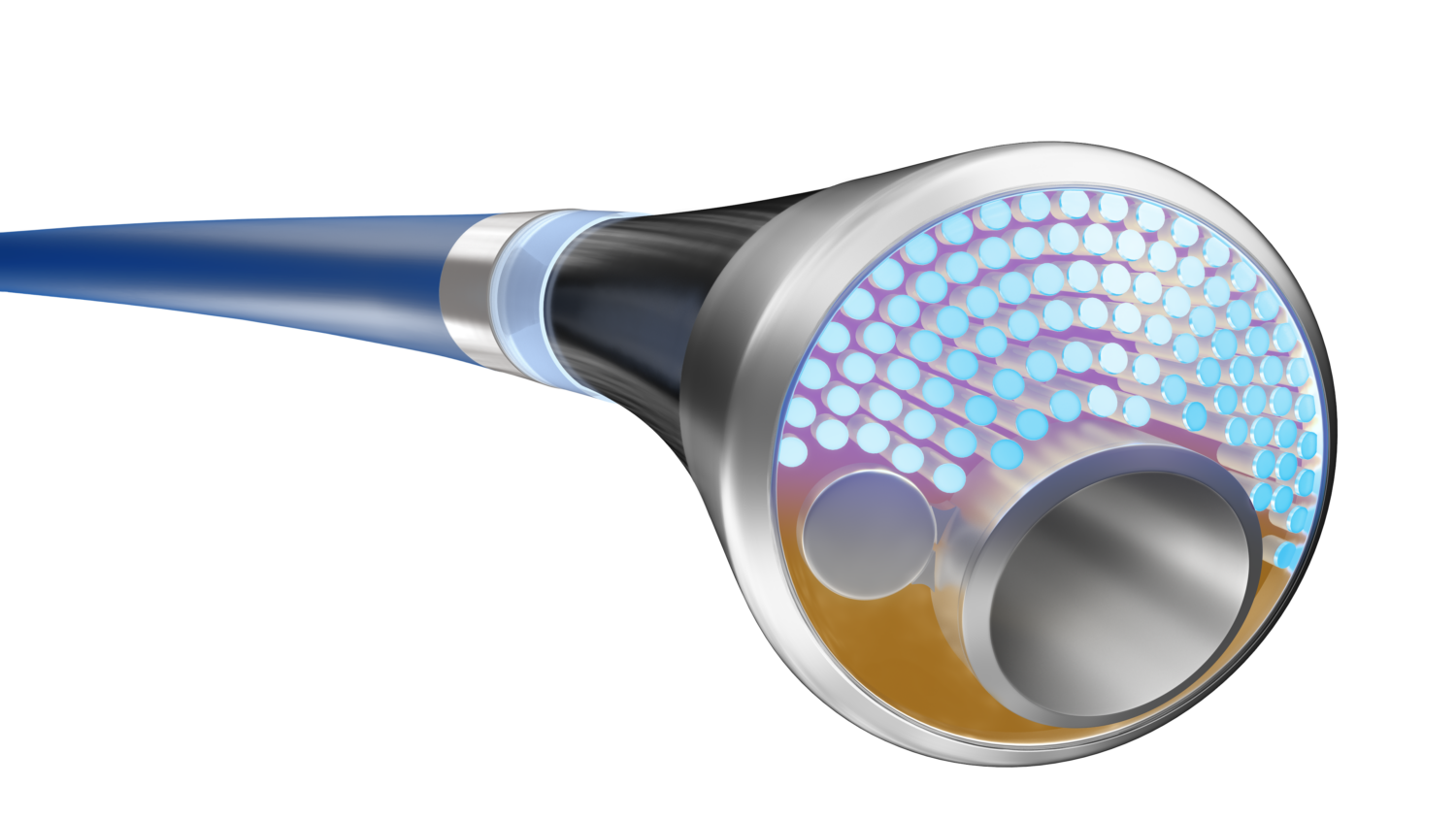
How the Philips Device Works
The Philips laser catheter uniquely merges laser atherectomy and intravascular lithotripsy—both essential for effectively treating PAD. Here’s how each component functions:
– Atherectomy: This technique removes atherosclerotic plaque from within the artery, restoring blood flow.
– Intravascular Lithotripsy: This treatment modifies or disrupts calcium deposits in the artery wall, addressing calcification that can block blood flow.
Unlike conventional intravascular lithotripsy devices, which use a separate ultrasound catheter to create shockwaves, Philips’ new device uses a pulsed laser that instantly vaporizes fluid within the blood vessel. This action produces expanding and collapsing bubbles that generate the required sonic waves to treat calcifications within the artery.
By combining these two therapies, the Philips catheter significantly reduces the complexity of PAD treatment workflows, allowing healthcare providers to treat complex, calcified lesions with a single device.
Details of the THOR IDE Trial
The THOR IDE trial is a prospective, single-arm, multicenter study that will enroll up to 155 patients across 30 sites in the United States. Conducted under an Investigational Device Exemption (IDE) granted by the U.S. Food and Drug Administration (FDA), the trial aims to assess the system’s safety and effectiveness in achieving procedural success with minimal complications.
The study’s primary endpoints include freedom from major adverse events (MAEs) such as mortality, unplanned amputations, and clinically driven target lesion revascularization (CD-TLR) within 30 days of the procedure. Additionally, the trial will assess the device’s ability to achieve less than or equal to 50% residual stenosis post-procedure, an essential measure of its efficacy. Each patient will be followed for 12 months after the procedure to monitor for potential long-term outcomes and improvements.
Implications for PAD Treatment and Patient Outcomes
Philips’ innovative laser catheter holds the potential to revolutionize the PAD treatment landscape. By integrating two treatments into a single procedure, it offers numerous advantages for both healthcare providers and patients:
– Simplified Workflow: By reducing the need for multiple devices, the Philips device simplifies the PAD treatment process, which can potentially reduce procedural times and hospital stays.
– Lower Risk: Combining two procedures into one reduces the risk associated with repeated interventions and lowers the chances of complications.
– Improved Patient Experience: Patients may benefit from fewer hospital visits and a shorter recovery period, which can enhance their overall quality of life.
Looking Ahead
The Philips laser atherectomy and intravascular lithotripsy device is currently investigational and not yet commercially available in the United States or worldwide.
