What You Should Know:
– Innoventric, a leader in transcatheter tricuspid regurgitation (TR) treatment, today announced a $28.5 million Series B funding round to advance its revolutionary cross-caval technology, bringing the total funds raised since inception to $41 million.
– Innoventric has already successfully completed a first-in-human clinical trial in Europe, and performed many additional implantations — treating over 40 participants so far. Recently, the company received FDA clearance for an Early Feasibility Study (EFS) in the US, and patient enrollment is actively ongoing with the first US patients already treated. The funds raised will be used to advance clinical trials and expand regulatory approvals in the US and Europe.
Innoventric: Pioneering Transcatheter Solutions for Tricuspid Regurgitation
Since its establishment in 2017, Innoventric has led advancements in transcatheter solutions to address the complex issue of tricuspid regurgitation (TR). Focused on cross-caval technologies, Innoventric prioritizes data-driven innovation, drawing from deep clinical research to deliver treatments designed for a wide range of patient anatomies. Its commitment to high-quality care aims to make effective TR treatment more accessible and improve patient health outcomes.
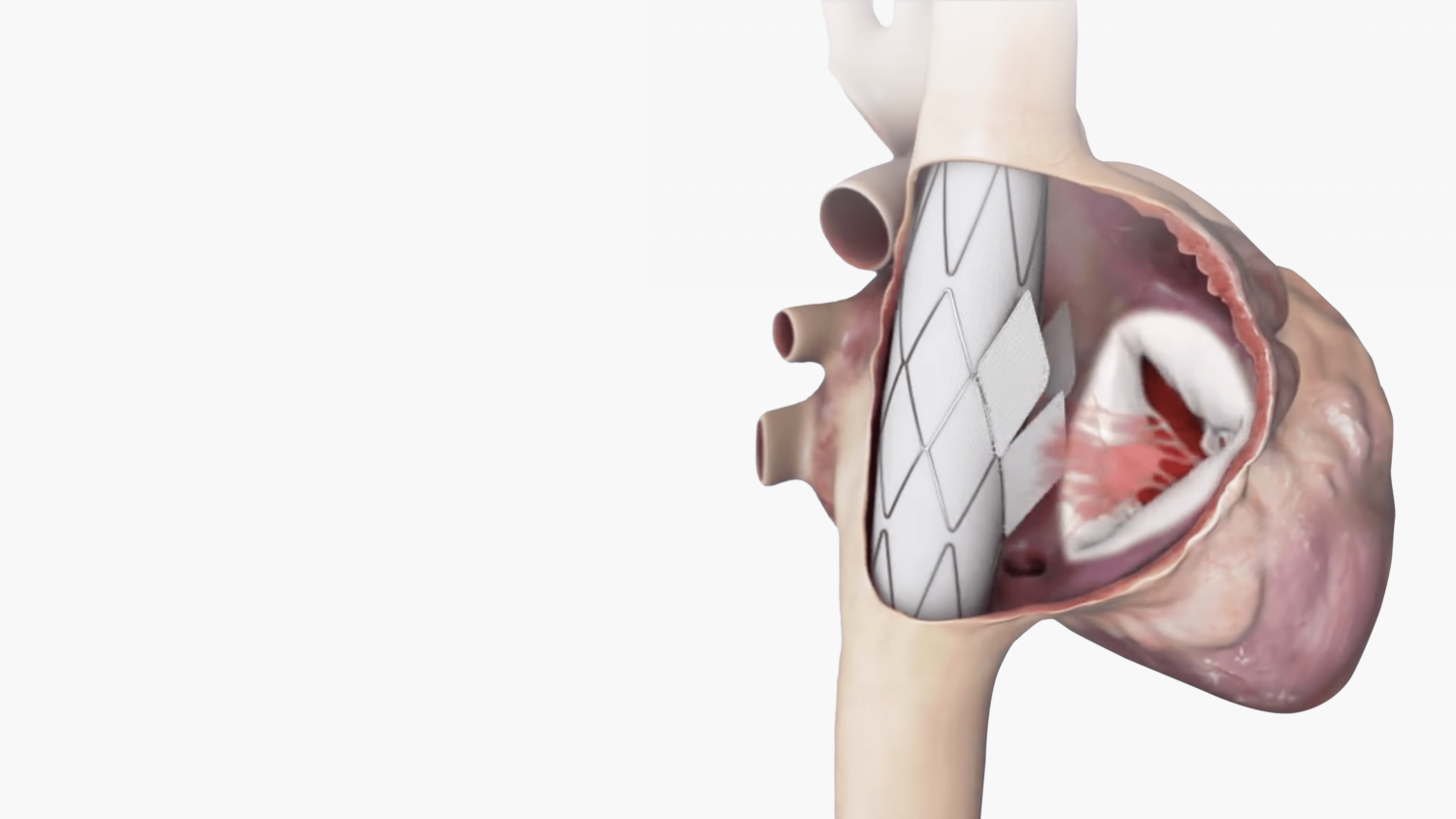
Innoventric’s flagship device utilizes a unique heterotopic, cross-caval approach, restoring function to the tricuspid valve without directly interacting with the heart. By securely anchoring a prosthetic valve to the superior and inferior vena cava (SVC and IVC), Innoventric achieves a complete seal, eliminating risks of leakage or detachment while simplifying the implantation process. This design effectively addresses the anatomical challenges typical of traditional TR treatments.
Key advantages of Innoventric’s device include:
– Broad Patient Applicability: By accommodating diverse anatomies, the device extends treatment options to patients who may not qualify for conventional tricuspid procedures.
– Innovative Anchoring Technique: Anchoring to the stable tubular structure of the SVC and IVC, rather than the heart, enhances security and minimizes complications, such as leakage and detachment.
– Streamlined Procedure: The device can be implanted quickly without the need for echocardiography or general anesthesia, improving procedural success rates and shortening patient recovery time.
Positioned at the forefront of the $10 billion annual transcatheter heart valve replacement market, Innoventric’s technology is poised to revolutionize tricuspid valve treatment. Supported by significant recent funding, the latest investment round was led by RA Capital Management and the European Investment Committee (EIC), with continued support from BRM Group, JG Private Equity, and Mivtach Shamir Holdings—demonstrating substantial confidence in Innoventric’s transformative technology.
Amir Danino, CEO of Innoventric, stated: “Our mission is to revolutionize tricuspid regurgitation care with minimally invasive therapies that significantly improve patient outcomes. The strong backing from our investors, coupled with the progress we’ve achieved, underscores the need and huge potential of our approach to treat TR.”
