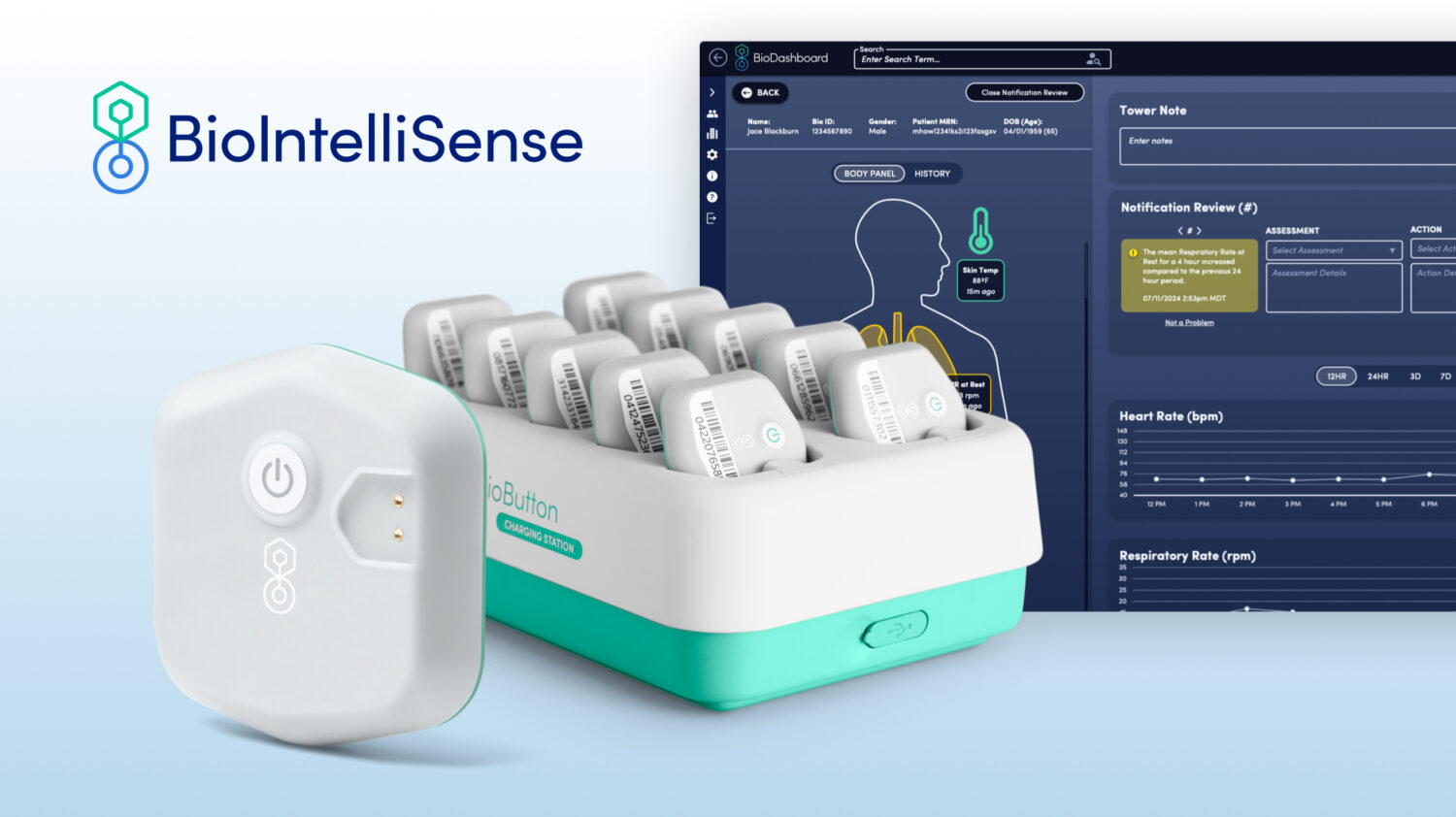
What You Should Know:
– BioIntelliSense, a leader in continuous health monitoring, has received FDA clearance for its rechargeable BioButton Multi-Patient wearable and BioDashboard system.
– The BioButton is poised to transform hospital virtual care programs by providing a cost-effective and scalable solution for continuous patient monitoring.
Rechargeable and Reusable Wearable
The BioButton Multi-Patient wearable is a game-changer for inpatient settings. This rechargeable and reusable device automates vital sign collection across various hospital departments, including medical-surgical units, specialty care areas, and emergency departments. It even extends continuous care to the home environment, facilitating hospital-level monitoring in a patient’s own residence.
Data-Driven Clinical Intelligence
The BioDashboard clinical intelligence system complements the BioButton by providing clinicians with a powerful tool for data analysis and decision-making. Its exception management approach delivers personalized, automated notifications based on each patient’s unique data trends. This allows a single clinician to effectively monitor hundreds of patients simultaneously, enabling proactive interventions and improved patient outcomes.
“We are advancing a new standard of care by automating the capture of thousands of multiparameter measurements each day and for every general care patient throughout their inpatient stay and now the BioButton Multi-Patient wearable is conveniently rechargeable with its own dedicated in-facility charging station,” said James Mault, MD, founder and CEO of BioIntelliSense. “The introduction of the rechargeable and reusable BioButton Multi-Patient wearable, combined with the BioDashboard clinical intelligence solution, provides health systems nationwide unprecedented economies of scale in making continuous care more affordable and accessible for every patient that is admitted to the hospital.”
