What You Should Know:
– 3Aware, a provider of medical device regulatory compliance solutions, and Mayo Clinic Platform, a collaborative ecosystem for healthcare innovators, today announced a new partnership that will integrate 3Aware’s aiSurveillance solution with Mayo Clinic Platform’s extensive clinical data resources.
– This collaboration marks a significant milestone in the industry as it will enable medical device manufacturers to automate post-market clinical follow-up (PMCF) and gain deeper insights into device safety and performance.
Enhanced Device Coverage and Automated PMCF
The integration of aiSurveillance with Mayo Clinic Platform will significantly increase the device coverage of 3Aware’s solution, enabling manufacturers to automate PMCF for a broader range of devices. By leveraging Mayo Clinic Platform’s comprehensive, longitudinal real-world data, 3Aware will provide manufacturers with deeper insights into device-specific patient experience and outcomes.
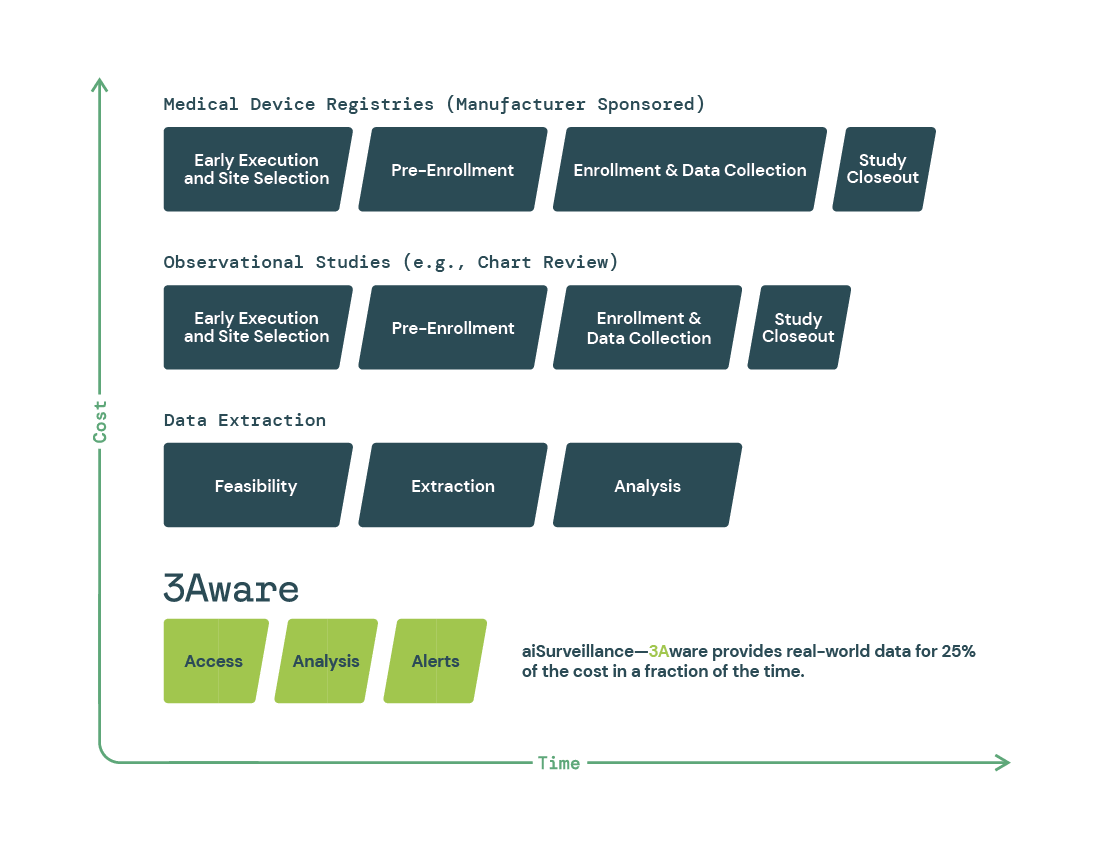
Reduced Cost, Time, and Risk of Regulatory Compliance
3Aware’s aiSurveillance solution has been shown to reduce the cost, time, and risk of MedTech regulatory compliance. By using active surveillance, manufacturers can achieve PMCF-based compliance at less than 50% of the cost and one-tenth of the time of chart reviews and registries. Additionally, aiSurveillance provides a deeper, patient-level understanding with less bias compared to traditional methods.
Access to Extensive Clinical Data
Mayo Clinic Platform provides unparalleled access to extensive, diverse, and de-identified clinical data, including structured data, such as demographics and diagnoses, and unstructured data, such as medical imaging and clinical notes. This data is continually updated and encompasses more than 3 billion images, 676 million clinical notes, 1.6 billion lab results, 9.7 million pathology reports, and 662 million clinical notes.
Data Behind Glass: Secure Access to De-identified Data
Mayo Clinic Platform’s Data Behind Glass model enables secure access to extensive sets of de-identified data without moving it among the contributing organizations across multiple continents. This unique approach ensures patient privacy while providing manufacturers with the data they need to gain valuable insights into device safety and performance.
