What You Should Know:
– Curebase, a company committed to democratizing access to clinical trials, announced the release of its integrated software and services package for digital therapeutics (DTx) trials that accelerates enrollment and allows sponsors to launch their studies faster.
– Available now, the new Curebase offering includes the first one-stop-shop decentralized clinical trial (DCT) platform bundled with site and trial execution services for the DTx market.
Making Healthcare Readily Available Via All-in-One Software and Trial Execution Package
This unique blend of eClinical software and technology-driven services enables sponsors of DTx studies to leverage the following Curebase capabilities:
Technology and virtual site models: The company’s fit-for-purpose DCT platform was developed to support remote and hybrid digital therapeutic trials by offering flexible virtual site models, extensive patient data capture capabilities, and experienced digital recruitment strategies.
Site services: Curebase’s in-house clinical research coordinators (CRCs) have deep experience interacting with patients for DTx studies as well as principal investigators (PIs) who have run successful studies for Curebase clients. This provides DTx trial sponsors, using the new Curebase offering, access to a valuable clinical studies knowledge base and investigators/virtual site staff experienced in digital therapeutic studies.
Start-to-finish execution: DTx logistics and details can be overwhelming to sponsors. As part of its DTx offering, Curebase provides trial management services, such as Institutional Review Board (IRB) submissions, project management, CRO services, data management, patient recruitment, and more. These exhaustive capabilities make it easy for DTx sponsors to find everything they need in one place to set up, recruit for, and execute digital therapeutic studies.
For most DTx sponsors, launching a study is a time-consuming and complicated process involving multiple components, such as finding a software vendor, locating sites, engaging a principal investigator, and clinical research organization (CRO). The new Curebase digital therapeutics offering includes features, workflows, and tools built into the company’s eClinical platform specifically made for and used in DTx studies. This also includes virtual site models, CRO services, and recruitment tactics tried and tested in digital therapeutic studies, allowing DTx sponsors to quickly launch a study with the confidence of proven execution.
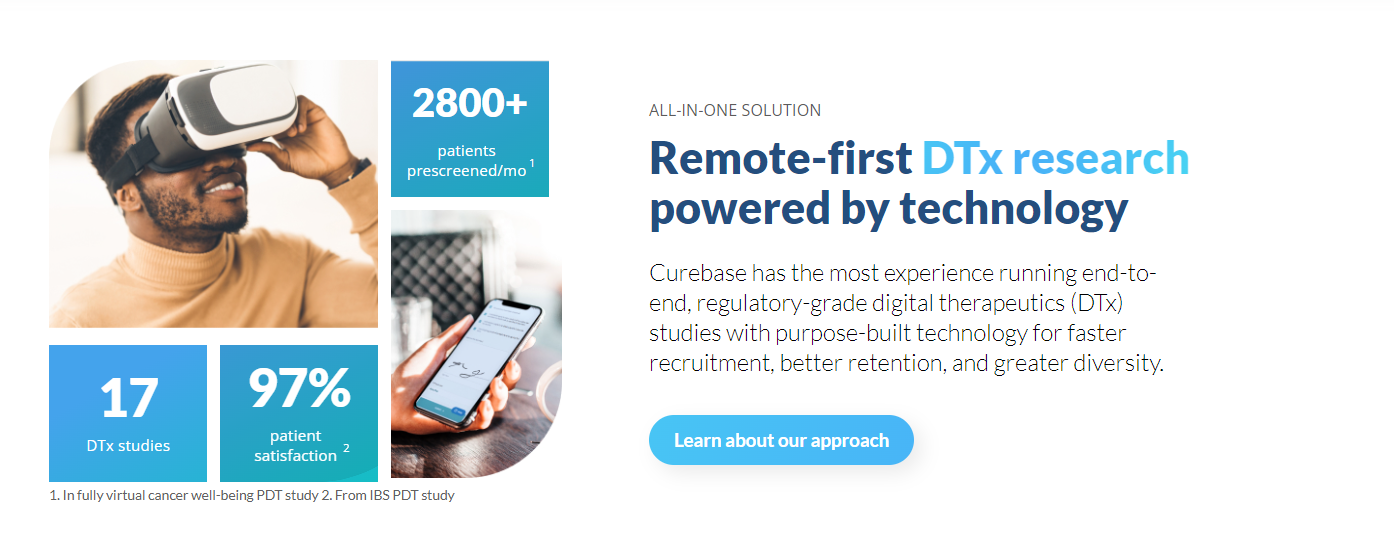
Curebase has completed 17 studies, enrolled more than 3,500 patients, and prescreened tens of thousands for DTx sponsors. These trials have tested DTx products in depression, anxiety, gastrointestinal problems, cancer, heart issues, rare diseases, and more. Much of this experience is evidenced and represented in Curebase’s membership and collaboration with the Digital Therapeutics Alliance (DTA), which is utilizing the company’s expertise to develop guidance and standards for DTx studies.
“Our new offering streamlines vendor selection and trial execution for DTx companies,” said Whitney Stewart, director of clinical project management at Curebase. “That translates to more trial completions and getting more products closer to the hands of patients.”
DTx products deliver evidence-based therapeutic interventions through software programs to treat, manage, or prevent mental health disorders, substance abuse, diabetes, chronic pain, and other physical or behavioral problems. These products can be used independently or with medications, devices, or other therapies to help patients attain better outcomes.
Not only does the Curebase DCT model accelerate the enrollment process, but it also allows DTx trial sponsors to scale studies to include more geographically and ethnically diverse groups of participants, which can lead to more effective digital therapeutics.
