What You Should Know:
– Oncoustics, San Francisco, CA-based ultrasound-based tissue characterization solutions announces the initial close of a $5 million+ seed round of funding to advance its SaMD (software as a medical device) technology for the low-cost assessment of structural diseases at the point of care.
– Oncoustics’ first products will focus on liver disease, one of the fastest-growing causes of morbidity and mortality in the world. The round is co-led by Creative Ventures and Saltagen Ventures, and further includes NorthSpring Capital Partners, Fraser Kearney Capital Corp., Pallasite Ventures, and Dr. Chen Fong, a renowned radiologist, entrepreneur/investor and inductee into the Order of Canada for his contributions to medical technology innovation and philanthropy.
Acoustic Data Meets Ultrasound Imagery
Oncoustics’ patented approach utilizes both the ultrasound images as well as the acoustic data derived from the raw sound signals to automatically differentiate tissue types. Every different type of tissue in the body bounces back a unique acoustic signature and Oncoustics mines these signals to differentiate healthy versus diseased tissues. Oncoustics has been collecting ultrasound signal datasets and has amassed the largest RF signal data set in the world. This hardware-agnostic approach works on any ultrasound system, including new low-cost point-of-care ultrasound systems, making this an affordable and accessible diagnostic tool.
James Wang, partner at Creative Ventures who leads AI investments, leveraging his background in computer science specializing in AI/ML, shared, “Oncoustics’ approach is unique. They mine the raw data that is typically thrown away and can use this to go beyond what can be seen by the human eye. They’re basically creating a ‘virtual biopsy’ that has vast applications in healthcare. Innovations like Oncoustics are what will help change the trajectory of our currently unsustainable healthcare system and costs.”
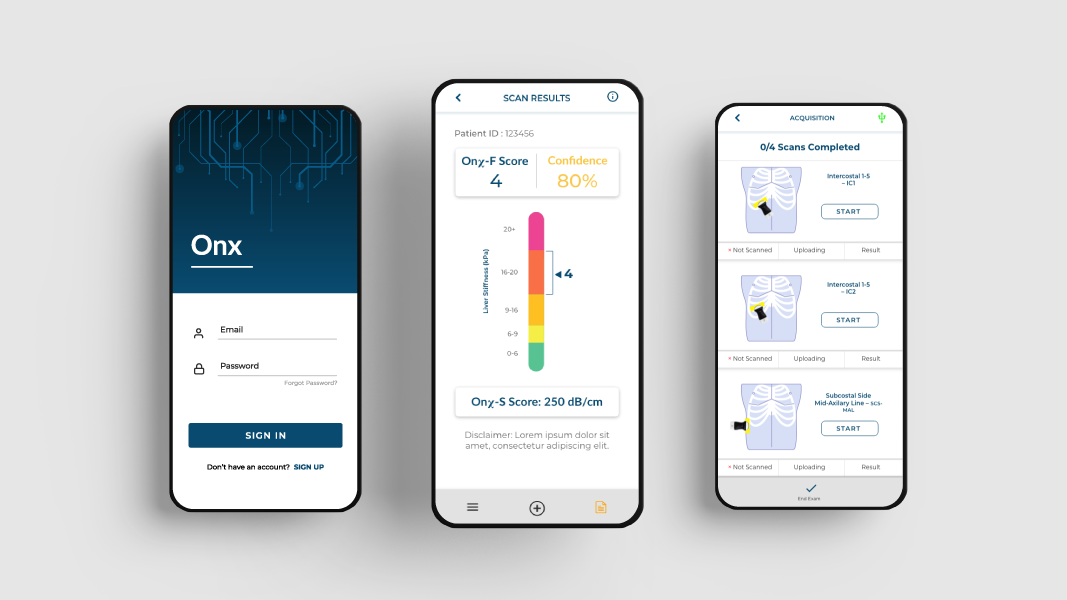
Leveraging the rise of new point-of-care ultrasound systems, Oncoustics takes advantage of all the benefits of these systems, including their low cost, portability and ease of use, and builds on this by guiding the data acquisition and providing easy-to-read results via a smartphone app.
Oncoustics’ Liver Assessment Solution
Oncoustics’ first product, the OnX liver assessment solution, is focused on detecting structural liver diseases including fibrosis and steatosis (fat) that can occur in all types of chronic liver disease (CLD). CLD today affects more than 2 billion people globally and is rising dramatically, driven by a condition called Non-Alcoholic Fatty Liver Disease (NAFLD).
“There’s a tsunami of need around detecting these types of liver diseases and our ultimate goal is to decrease or eliminate the need for high-end imaging or painful and invasive biopsies,” said Beth Rogozinski, CEO, Oncoustics. “With this new round of funding, we will accelerate our liver solutions and enable low-cost diagnostics for earlier interventions and better patient care.”
Milestones
The Oncoustics platform promises a whole new level of access to care with the benefits of ease of use, accessibility, affordability, and optimizing clinical workflow. Oncoustics applies AI to raw ultrasound signals to do tissue characterization at point of care for low-cost, noninvasive surveillance, diagnostics, and treatment monitoring of diseases with high unmet clinical need. The Oncousticssolutions for ultrasound will be submitted for regulatory approval in the United States (FDA 510(k)), Canada (Health Canada medical device license) and the European Union (CE Mark). The OnX Liver Assessment Solution has not been cleared for clinical use and is For Investigational Use Only.
