What You Should Know:
– Mammoth Biosciences signs agreements with MilliporeSigma and Hamilton company targeting commercialization of high-throughput CRISPR-based SARS CoV-2 Test.
– These partnerships will help Mammoth bring a turnkey CRISPR-based sample-to-answer solution for commercial laboratories to enable a multi-fold increase in a testing capacity.
Mammoth Biosciences, Inc., announced that it has signed agreements with MilliporeSigma and Hamilton Company targeting commercialization of a high-throughput CRISPR-based SARS CoV-2 test. The test leverages Mammoth’s DETECTR BOOST™ platform and will provide a sample-to-answer turnkey solution for commercial laboratories to enable a multi-fold increase in testing capacity.
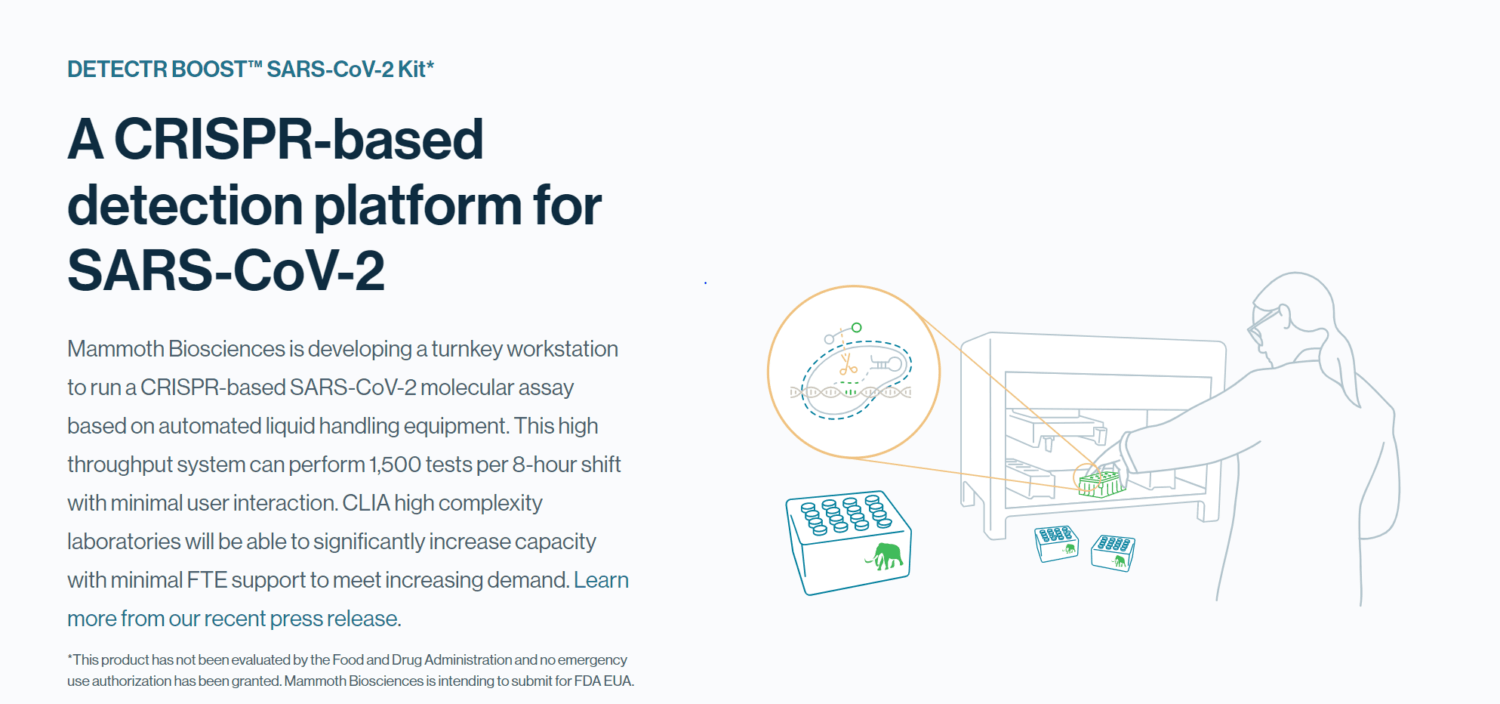
As laboratories nationwide face limited capacity and testing supply shortages amid the pandemic, Mammoth Biosciences is poised to help reduce the backlog by working with leading manufacturing and automation vendors for its CRISPR-based SARS-CoV-2 molecular assay. The high-throughput systems will be compatible with both nasal swab and saliva samples and are targeting 1500 tests per 8-hour shift with minimal user interaction. Mammoth Biosciences plans to submit the assay for FDA Emergency Use Authorization later this year.
“In order to begin reopening more aspects of society, we’ll need a robust testing infrastructure that can rapidly scale up capacity as needed,” said Trevor Martin, Ph.D., co-founder and CEO of Mammoth Biosciences. “By combining CRISPR-based diagnostics with the proven manufacturing and product leadership of MilliporeSigma and Hamilton Company, we’re confident this solution will be a game-changer for labs.”
Mammoth’s DETECTR BOOST™ SARS-CoV-2 assay reagent kits, which will be contract manufactured by MilliporeSigma, rival PCR in sensitivity while being less prone to supply chain risk. The testing system will also leverage standard, automated liquid handling equipment such as those from Hamilton Company to enable rapid processing of patient samples. By implementing this unique offering, CLIA laboratories will be able to significantly improve their capacity to regularly test communities and businesses in need.
“This collaboration will allow us to help Mammoth with the development and production of their new SARS-CoV-2 test, which, once approved by the FDA, will increase testing capacity here in the U.S. at the scale needed to combat this pandemic,” said Jean-Charles Wirth, head of Applied Solutions at MilliporeSigma. “MilliporeSigma is helping to advance the future of testing at scale. This is an important step in detecting Covid-19 quickly.”
