What You Should Know:
– Medable launches Teleconsent, part of its Telehealth family of cloud-based software for decentralized clinical trials.
– The application represents the next big step in enabling fully virtual trials and is key to patient-centric trial design because it allows patients to handle the lengthy process of consenting – and re-consenting – to trial participation conveniently from anywhere.
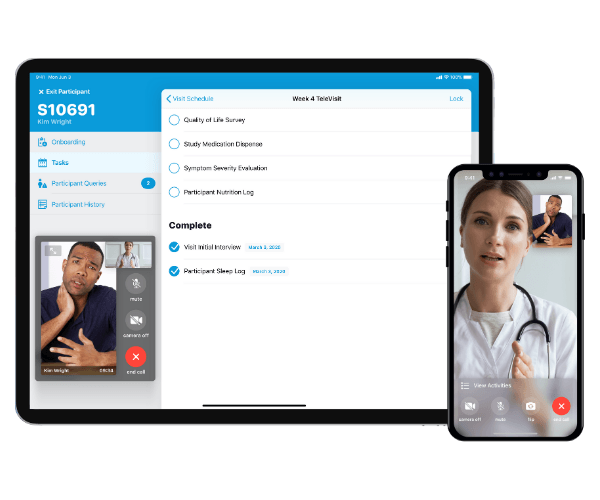
Medable Inc., a software provider for decentralized clinical trials, today announced general availability of Medable TeleConsent™, a new product that enables fully remote informed consent and re-consent for clinical trials. Unlike traditional eConsent products that require both patient and investigator to be physically present together in the clinic, Medable TeleConsent allows patients, doctors, nurses, and clinical trial staff all to connect and sign remotely from any location.
Available immediately, Medable TeleConsent was designed specifically to power several modalities of decentralized trials—and is the first eConsent application that enables patients, sites and sponsors to all engage remotely via web or mobile device in multiple languages.
Medable TeleConsent: The Next Piece of the Decentralized Trial Puzzle
Medable TeleConsent solves one of the most complex aspects of clinical trials for sites and sponsors—and transforms the initial experience for patients. By eliminating the need for multiple round-trip visits to clinical sites, TeleConsent dramatically improves patient access to studies, connecting them directly with trial investigators and site teams from their home location. This results in faster enrollment, greater participant diversity and better retention for trial sponsors.
TeleConsent also improves patient knowledge and comprehension by providing medical information in visual and multimedia formats, which patients can review in depth together with family members and caregivers. They can then engage visually with their physician to sign consent forms digitally from the comfort of their home or local clinic.
Why TeleConsent Is Critical During COVID-19
Medable TeleConsent is especially critical in the COVID-19 environment, where many patients are staying home to avoid social interaction and minimize exposure. Sites and sponsors can now screen, enroll and consent study participants without meeting in person, taking advantage of Medable’s TeleVisit application to conduct personalized interactions that improve patient understanding. Sites and sponsors benefit from streamlined workflows and enhanced data quality and compliance. Sponsors also get increased transparency with real-time reporting and insight into study progress. TeleConsent can also be used for re-consenting patients for any future changes that may happen in a clinical trial.
Design Approach
Medable TeleConsent was designed uniquely with a patient-centric perspective, informed by Medable’s Patient Advisory Council, a nationwide network of advocates who advise Medable and customers on ways to improve patient access, experience, and outcomes.
Medable TeleConsent is designed to be flexible, so the features can adapt to local regulatory, site and patient-preferred workflows. This enhances the experience while ensuring clear and accurate dissemination of critical information. TeleConsent also records digital evidence of comprehension and knowledge transfer, while capturing investigator and patient agreements to proceed. Having access to digital records is valuable for all stakeholders and sets the foundation for ongoing digital engagement between sites and patients.
“Medable TeleConsent is a critical step in the evolution of decentralized research, as consent is the gateway to trial participation,” said Dr. Michelle Longmire, CEO and co-founder of Medable. “This has the potential to enable global patient access and improved knowledge, leading to greater participant diversity, retention, and ultimately research quality and speed. We’re excited to break down these barriers to benefit patients, sites and sponsors alike.”
