Vixiar Medical, Inc., a Baltimore, Maryland-based company dedicated to the development of non-invasive devices and systems for monitoring cardiopulmonary diseases has raised $1.5 million from new and existing investors. New investors joining in this round included Emerald Development Managers, MMG Opportunities and several individual investors. Existing investors included The Maryland Technology Development Corporation (TEDCO) through its seed fund, the Abell Foundation, individual investors and management. The company plans to use the funding to complete an ongoing clinical trial and to prepare regulatory submissions.
Vixiar Medical Overview
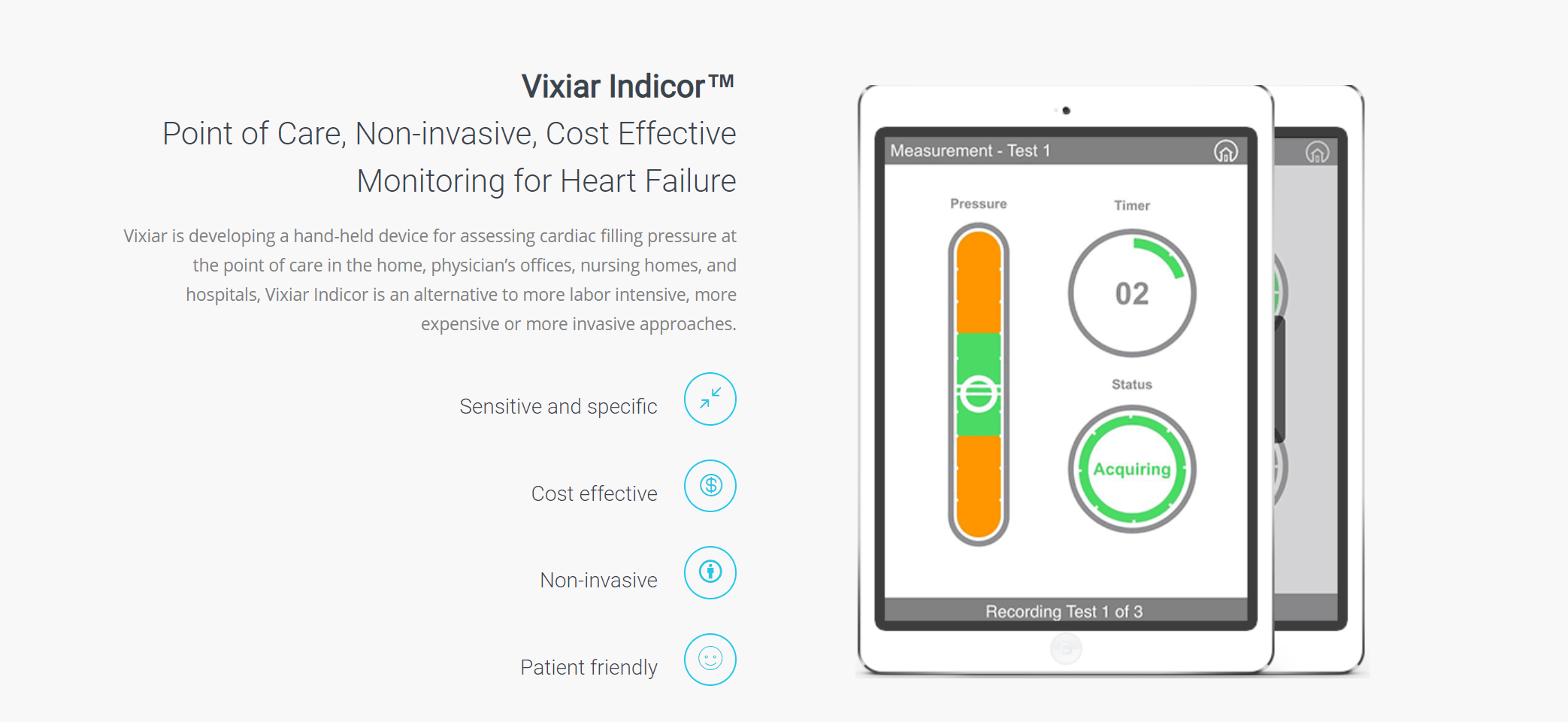
Founded in 2014, Vixiar Medical is a spinout of John Hopkins that develops non-invasive, cost-effective devices and systems for monitoring cardiopulmonary diseases, particularly those with the significant clinical and economic burden. The company’s first product, Indicor™, is a handheld point of care device and digital platform for monitoring worsening heart failure.
Funding Impact
“We are pleased to welcome new investors to this round and with the continuing commitment from existing investors,” said Vixiar CEO, Kevin Thibodeau. “Heart failure is a large and growing global concern and there is currently a lack of non-invasive, point-of-care technologies for addressing the major problem of costly hospitalizations and readmissions. Our technology offers a rapid, non-invasive, cost-effective device designed to be used across the continuum of care including hospitals, physician offices, skilled nursing facilities, and patient homes.”
