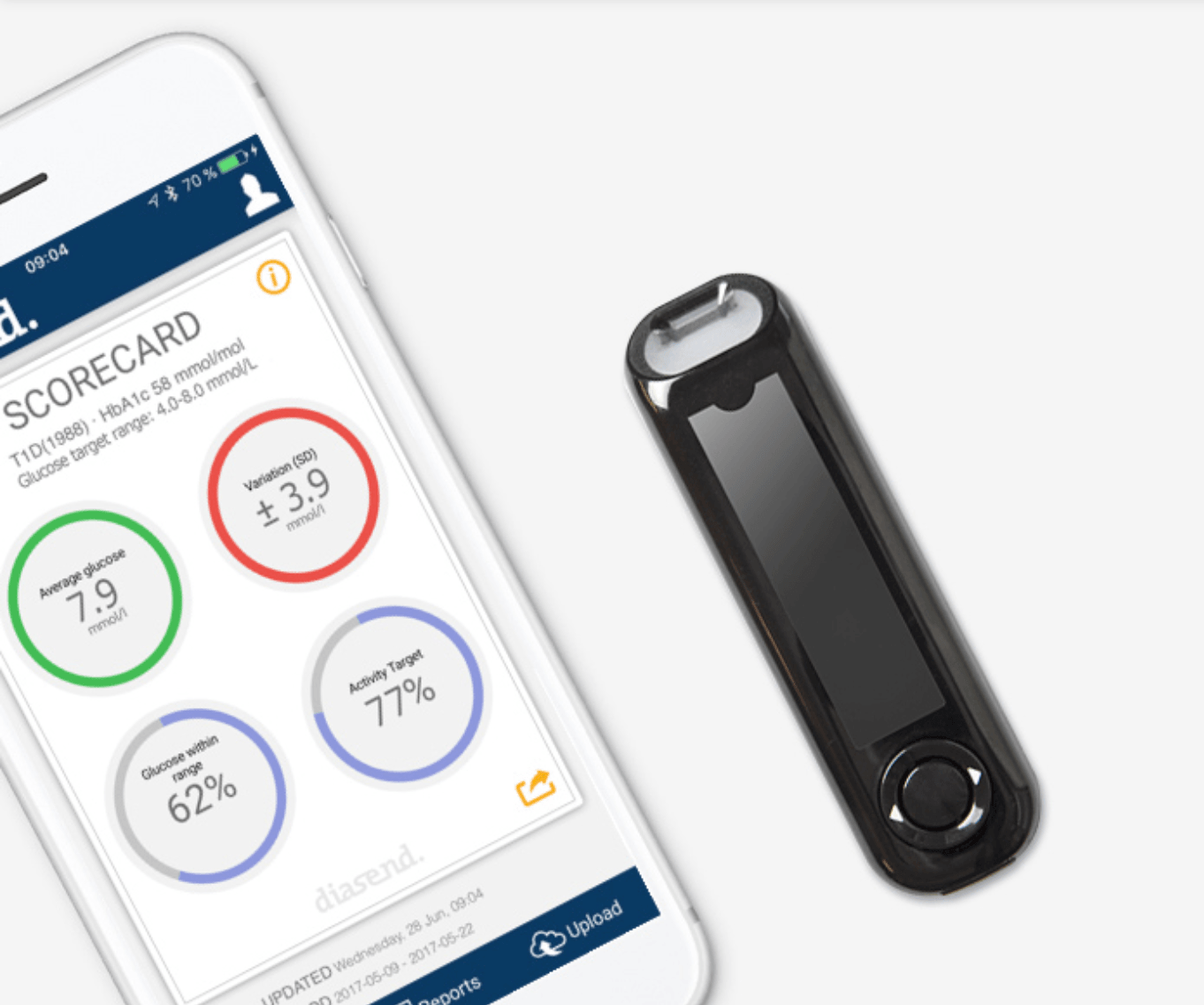
Today, Glooko announced the launch of their integration with the Novo Nordisk connected insulin pen on our diasend® platform and the release of their Annual Diabetes Report. With diasend, doctors were able to see and advise patients based on recorded insulin dose data from connected Novo Nordisk pens without having to invest additional time. Now, to raise the bar for patient convenience even further, Glooko is launching the ability to also upload pen data remotely – straight from any near field communication (NFC) equipped Android phone running the diasend Mobile App.
Novo Nordisk’s Insulin Pen Availability
The launch of Novo Nordisk’s first connected insulin pens, NovoPen 6 and NovoPen Echo Plus, will commence in early 2019. Any healthcare provider using Glooko’s diasend product in the clinic, and any patient using the diasend Mobile App on an NFC equipped Android phone, will be able to sync data from the pens.
“This is a big step in using data to improve diabetes management. Until now, data from insulin pens was self-reported and subject to error, or often simply not captured. Our partnership with Novo Nordisk enables this insulin data to be seamlessly synced to diasend®, where patients and providers can easily see the data correlated with glucose levels, activity information and more. This allows much more informed conversations and decisions about the insulin regimen as well as other aspects of the treatment plan” says Russ Johannesson, CEO of Glooko.
Compatibility with Dexcom’s CGM Solution
Glooko is also rolling out compatibility with Dexcom’s popular G6 continuous glucose monitoring system for the diasend® solution. This means that PWDs can either upload data to diasend® when visiting their health care provider, via the in-clinic upload solution*, or sync continuously by setting up a cloud-to-cloud connection with their Dexcom CLARITY account. The latter is now also possible through the Glooko product, offered in the US.
Glooko Annual Benchmark Report Overview
In addition, using the over 15B health data points at Glooko, the company has released their annual benchmark report that uncovers information like which country has the lowest average blood glucose value, countries with the highest frequency of hypoglycemia and which day was the best (most time in range) day for people living with diabetes.
