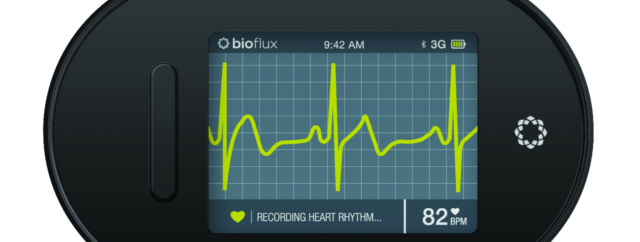
biotricity, a Redwood City, CA-based healthcare technology company has received a 510(k) clearance from the U.S. Food and Drug Administration (FDA) for a key component of its remote cardiac monitoring software and device, bioflux. Available only by subscription, bioflux will monitor a patient’s heart rhythm and send data in real-time to a 24-hour monitoring lab. If a patient is in cardiac distress, an alert will be sent to the monitoring center, which will in turn contact the patient to offer assistance.
This FDA clearance marks a major milestone for biotricity in their path to commercialization to develop multiple solutions for a variety of chronic illnesses by designing monitoring devices paired with chronic care management tools. Upon commercial availability, the device will be used by physicians and hospitals in the diagnostic process and then by patients for long-term care management.
This is particularly important because traditional healthcare diagnostic solutions are generally restricted to the physician’s office or inside of the hospital. bioflux’s software is designed to integrate seamlessly into a physician’s practice and workflow with no changes to internal processes.
“Achieving an FDA clearance validates the capability of biotricity to meet its vision of developing a series of clinically accurate medical devices that are applicable in both medical and home-based settings. Receiving a 510(k) clearance is a significant accomplishment towards our goal of enabling physicians to remotely monitor and diagnose patients with cardiovascular disease and coronary heart disease. More importantly, it is yet another step to making this vision a reality,” said Waqaas Al-Siddiq, Founder and CEO of biotricity in a statement.
“While wrist-worn fitness devices are incredibly popular and valuable for the fitness and lifestyle markets, they are not applicable for chronic patients. The reality is they are not cleared by the FDA, and we believe some of them may not be capable of reliably measuring heart rates,” said Al-Siddiq. “Unlike these light weight lifestyle/fitness devices, we aim to impact the healthcare by targeting at-risk patients and to do that you need medically relevant devices.”
AT&T, Inc. recently partnered with biotricity to serve as biotricity ‘s preferred network to power these devices with near real-time connectivity for data transmission. AT&T is currently already providing a pilot for connectivity based on the pending launch of biocricity’s flagship, the bioflux solutions by year-end.
