AirStrip announced it has received FDA 510(k) clearance to a web client for the AirStrip ONE® mobile interoperability platform and app that can be run on desktops and laptops using Internet Explorer and Google Chrome. AirStrip ONE® provides a single, data- and vendor-agnostic platform that connects clinicians with the right information at the right time, via the most convenient device at hand.
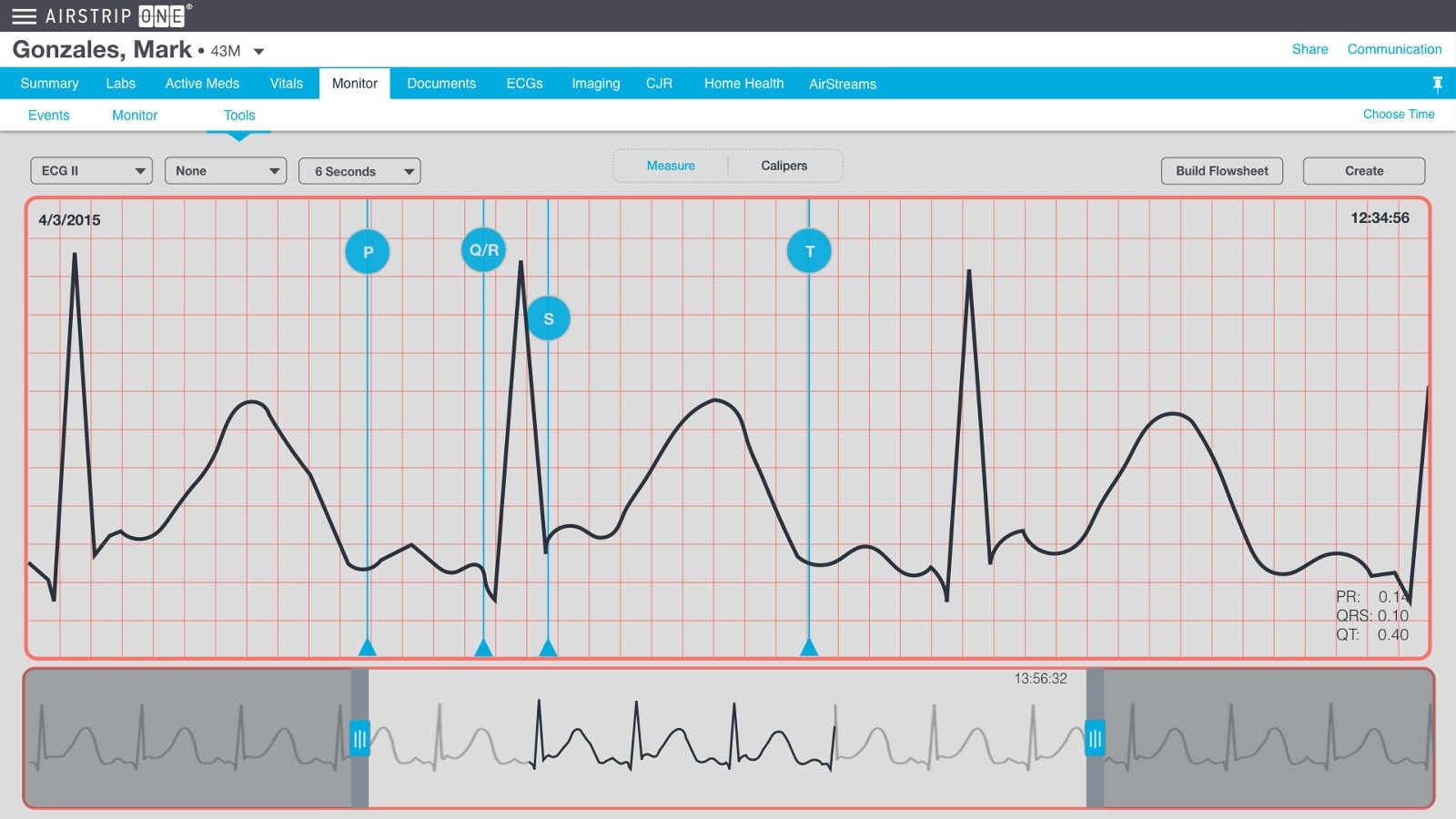
The company also recently received a U.S. patent for new functionality: ECG waveform ‘visual calipers’ within AirStrip ONE that allow clinicians to make measurements on digital waveforms, combine measurements, create waveform snippets and append documentation.The information collected using AirStrip ONE with visual calipers can then be constructed into a single document or booklet to document more complex events, which can be exported into the EMR. Structured data elements can also be sent into the EMR or a document management system.
Clinicians can quickly and accurately select the relevant ‘pre-, during, and post-‘ snippets during a complex cardiac event, supporting faster and more informed cardiac care decisions. Visual calipers can also be used to perform measurements without sending any information to an EMR.
“The visual calipers and snippets functions promise to have a substantial impact not only on tele-ICU and tele-CV services, but also on clinical workflows for alarm management,” noted AirStrip Chief Development Officer, JF Lancelot. “Time spent on documentation can be reduced and more precise information about the patient can be made available more quickly in the EMR. In addition, clinicians can reduce the time spent on non-clinical tasks such as finding, scanning, clipping and transporting paperwork.”
ECGs are commonly taken for the diagnosis of various classes of patients, providing a host of information to analyze cardiovascular health, including potential cardiac disease and arrhythmia. Nurses and telemetry technicians currently carry the burden of documenting baseline ECGs for patients at every shift. Instead of having to physically cut and tape paper ECGs for placement in charts or scanning into the EMR, AirStrip ONE with visual snippets enables this task to be automated.
