On Wednesday, the FDA announced the launch of an open source platform for community sharing of genomic sequencing data called precisionFDA. DNAnexus, the provider of cloud-based genome informatics and data management was awarded a research and development contract by the FDA to build the platform. precisionFDA is the FDA’s answer to its role under the White House’s Precision Medicine Initiative is to review the current regulatory landscape and develop a streamlined approach to evaluating next-generation sequencing NGS-based diagnostics.
precisionFDA Overview
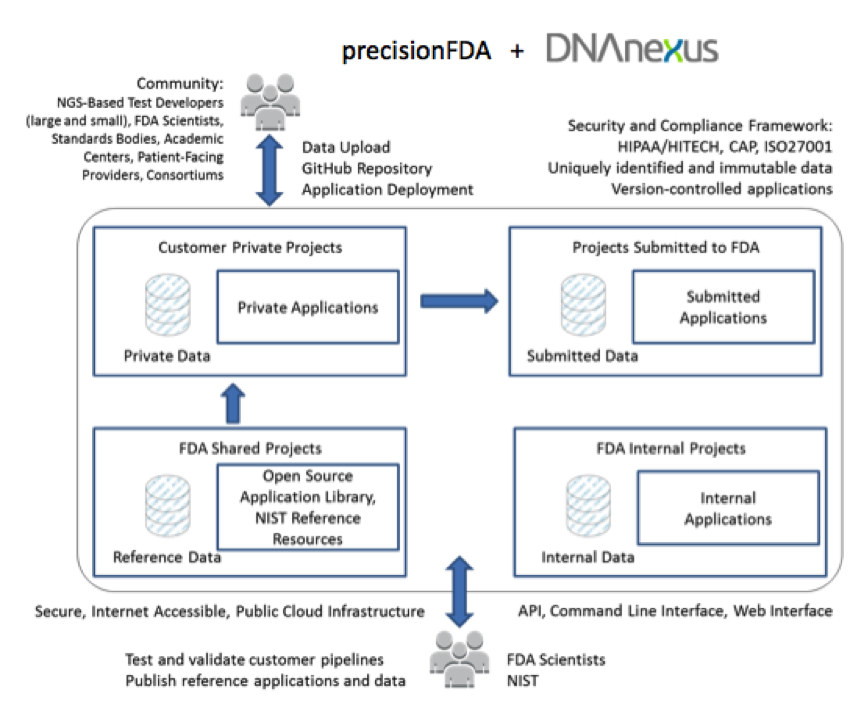
precisionFDA will crowd source reference analytical pipelines and datasets for the testing validation process by the community members who will be utilizing them. The DNAnexus Platform will deliver precisionFDA, providing the underlying cloud-based compute and data management infrastructure. In addition, DNAnexus will work with the FDA to build a community around its informatics platform to help drive standards around secondary analysis, the process of mapping, alignment, and variant calling of DNA sequence data. DNAnexus expects that the platform will be broadly used by NGS-based test providers, standards-making bodies, pharmaceutical and biotechnology companies, health care providers, academic medical centers, research consortia, and patient advocacy groups.
Key Objectives
In a post written by Taha Kass-Hout, MD, FDA’s chief health informatics officer, the key objectives for precisionFDA include:
– Exploring the use of a cloud-based portal, precisionFDA, to create a community around open-source genomic analysis pipelines, reference data, and analytical processing resources.
– Determining appropriate and auditable levels of security, privacy, and governance control to ensure the protection of collaborators’ intellectual property and protected information, while enabling interaction within the community.
– Providing an initial set of reference genomic data models and reference analysis pipelines.
– Independent genomic analysis and data management work areas that can be kept private or shared with owner’s choice of collaborators, the public, or FDA for vetting or validation .
“DNAnexus is proud to be delivering precisionFDA and creating a community around open-source genomic analysis pipelines, reference data, and analytical processing resources,” said Richard Daly, CEO of DNAnexus. “The FDA has taken a leadership position in making President Obama’s Precision Medicine Initiative a reality, and the DNAnexus platform will enable the managing and sharing of genomic data at an unprecedented level.”
