Guardant Health, a Redwood City, CA-based company revolutionizing cancer detection through biopsy-free tumor sequencing has raised $100M in Series D funding led by healthcare-investment firm OrbiMed. Existing Guardant Health investors Khosla Ventures, Sequoia Capital, Lightspeed Venture Partners, Pejman Mar, Formation 8, Heritage Group, and others also participated in the funding round.
Founded in 2012 by a team of serial entrepreneurs with expertise in next-generation sequencing, single-cell genomics and cancer diagnostics, Guardant Health will utilize the funding to accelerate their reach of its biopsy-free sequencing platform for cancer, Guardant360®. Guardant360 helps physicians identify genomic alterations in patients with advanced cancers, without the cost, risk, or pain of invasive biopsies. The 70-gene blood test is used in advanced cancer patients with visceral solid tumor cancers or metastases, and interrogates all four types of genomic alterations.
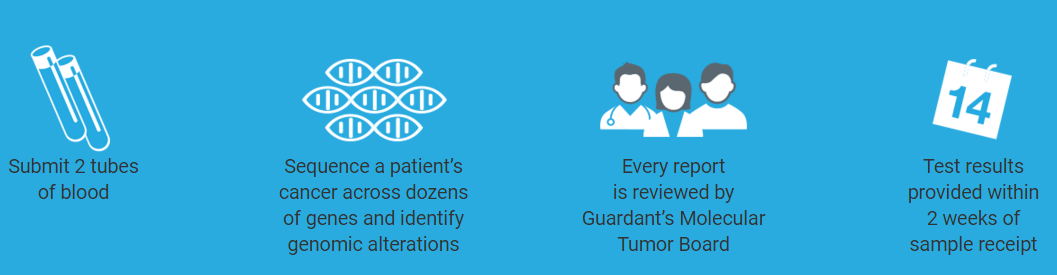
The test is used to prevent repeat invasive biopsies when cancer has progressed or recurred despite treatment, or when an initial biopsy is unobtainable or has insufficient tissue. It is the only ctDNA test that includes all guideline-recommended somatic genomic targets in a single test. Unlike hotspot tests, Guardant360 sequences complete exons so as not to miss uncommon or rare mutations. Based on the tumor genomic profile, clinicians receive a report of actionable genomic alterations and a list of FDA-approved treatments and clinical trials for which the patient could be eligible.
Guardant Health will also use the funds to further expand its Digital Sequencing™ platform into new product lines that address the complete cancer-care continuum.
“Genomic technologies are on the cusp of fundamentally changing cancer care. As new targeted cancer therapies emerge, our platform becomes that much more powerful. Oncologists already recognize our value; increasingly, investors do as well. This new funding is a strong vote of confidence in our technology, science, commercial, and reimbursement operations from one of the most sophisticated healthcare investors in the world. We will use it to help as many patients as we can to proactively combat cancer,” said Helmy Eltoukhy, Guardant Health CEO in a statement.
