The demand for clinical trial testing is rising, from over 260,000 trials conducted in 2018 to nearly double that figure in 2024. This means that more trials are being conducted simultaneously than ever—a factor that adds to the already challenging process of finding clinical trial participants.
Because trial sponsors are struggling to find sufficient numbers of suitable participants, 80% of the world’s more than 450,000 clinical trials are at risk of
Read More
Clinical Trials | News, Analysis, Insights - HIT Consultant
5 Ways Tech is Enhancing Patient Engagement in Clinical Trials
Patient engagement is critical on several levels. It helps inform updates to trial design and processes, it ensures critical data is collected to determine the safety and efficacy of an investigational product, and it supports patient retention for the duration of the trial. That’s why biopharma sponsors and research sites are leaning into technology to facilitate this engagement. It’s important for physicians and other healthcare professionals to be aware of this technology and how it can
Read More
FCS Partners with Paradigm to Expand Clinical Trial Access
What You Should Know:
- Florida Cancer Specialists & Research Institute (FCS), a leading provider of comprehensive cancer care, has announced a new partnership with Paradigm Health, a technology platform specializing in clinical trial management.
- The collaboration aims to expand patient access to innovative treatments through more efficient clinical trial recruitment and execution.
A Strategic Partnership for Enhanced Clinical Trial Access
FCS has experienced steady
Read More
Harvard Apparatus Regenerative Technology Secures $5M to Continue Clinical Trial
What You Should Know:
- Harvard Apparatus Regenerative Technology, Inc. (OTCQB: HRGN), a clinical-stage biotechnology company focused on developing organ regeneration technology for severe diseases, has secured approximately $5 million from a new investor through a private placement of shares.
- The funds will be allocated to expedite the clinical development of HRGN's lead product candidate, the HRGN Esophageal Implant (BEI).
- The FDA has approved a phase one and phase two clinical
Read More
Fujitsu Partners with Paradigm for Enhanced Clinical Trials
What You Should Know:
- Fujitsu today announced a series of initiatives aimed at attracting global clinical trials to Japan and addressing the issue of drug loss.
- By partnering with pharmaceutical companies and medical institutions, Fujitsu is leveraging its expertise in technology and healthcare to create a more efficient and effective clinical trial environment.
Key Initiatives
Partnership with Paradigm: Fujitsu has formed a strategic partnership with Paradigm Health,
Read More
Jumo Health Secures Funding to Improve Clinical Trial Enrollment
What You Should Know:
- Falfurrias Management Partners (Falfurrias), a Charlotte-based private equity firm focused on growth-oriented, middle-market businesses, today announced its investment in Jumo Health, an innovative and award-winning clinical trial solutions company driving better outcomes for clinical trials globally.
- Jumo Health develops creative, patient-centric educational solutions that improve health literacy to accelerate clinical trial enrollment and increase participant
Read More
The Role of AI in Streamlining Drug Discovery and Clinical Trials: A New Era in Pharmaceutical Innovation
In the rapidly evolving world of pharmaceuticals, artificial intelligence (AI) is emerging as a game-changer. From accelerating drug discovery to enhancing the efficiency of clinical trials, AI is revolutionizing how new medicines are brought to market. This transformation is not just limited to the research and development phases; AI is also playing a critical role in ensuring that drugs meet regulatory standards.
The AI Revolution in Drug Discovery
Drug discovery has traditionally been a
Read More
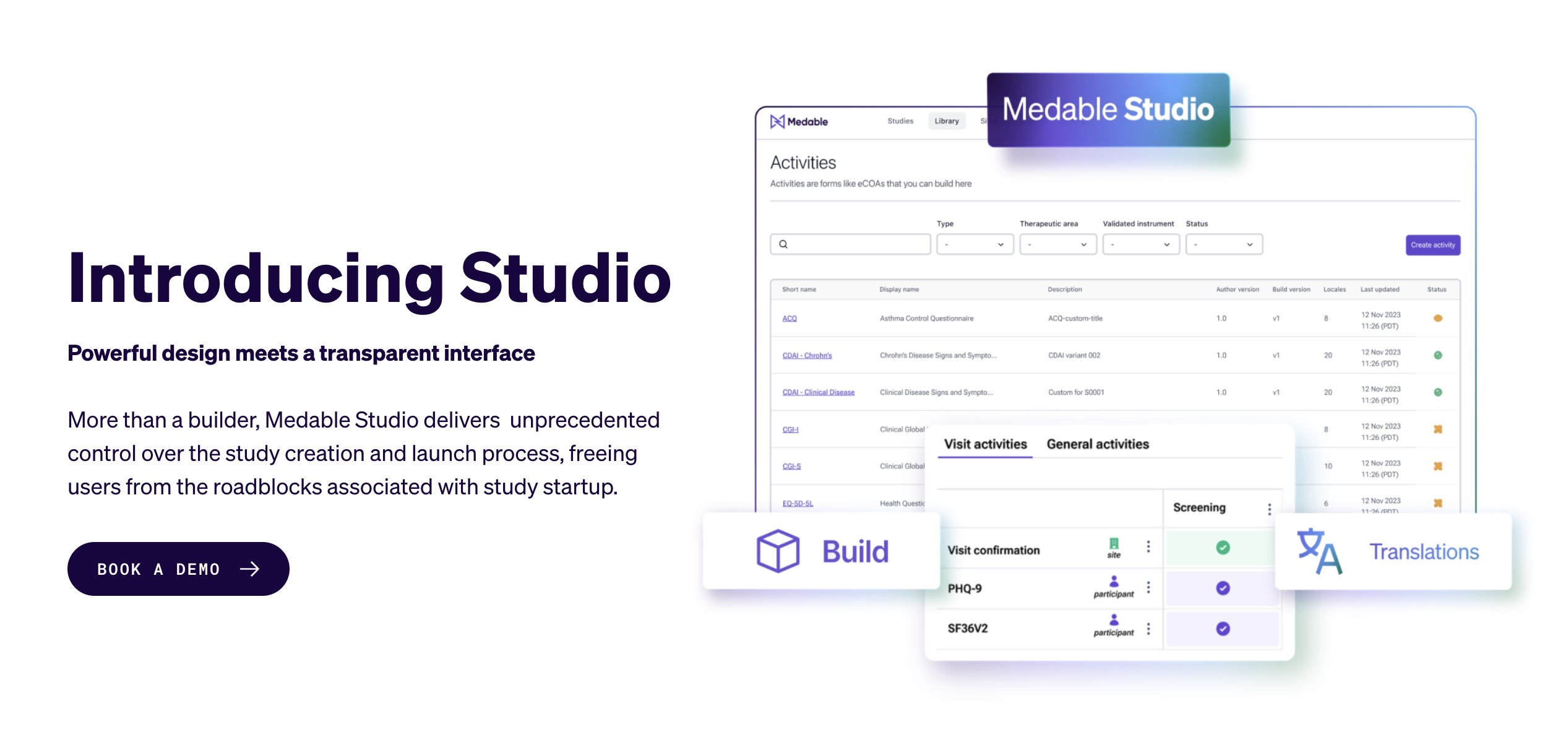
Medable Launches No-Code Platform for Clinical Trials
What You Should Know:
- Medable Inc., the leading technology platform for clinical trials, today announced Medable Studio, an all-in-one application for configuring, translating, validating, and launching eCOA Plus (eCOA, eConsent, Televisit, Sensors) into clinical trials.
- Studio is a no-code suite that simplifies the complex eCOA launch process, giving biopharmaceutical companies greater control and transparency for faster study go-live and earlier patient enrollment. Customers choose
Read More
Citeline Launches AI-Powered Tools for Clinical Trials
What You Should Know:
- Citeline, a provider of intelligence solutions for the life sciences industry, today announced the launch of Protocol SmartDesign and Investigator SmartSelect, two new AI-powered tools designed to streamline and optimize clinical trial development.
- These innovative solutions are part of the Citeline SmartSolutions suite, which leverages the company's extensive data assets to deliver actionable insights for life sciences organizations.
How Protocol
Read More
Clinical Trials Must Be More Equitable, Here’s How
The Need to Address Accessibility Gaps
Clinical trials are a driving force of medical innovation. However, they are predominantly conducted in large urban hospitals that have larger staff and more resources, which puts trial participation out of reach for the majority of Americans who live outside of major city centers. Despite having as much interest as city-dwellers, these individuals often find participation in clinical trials nearly impossible due to daunting travel, logistical and
Read More