What You Should Know:
- Sheba Medical Center, a leading Israeli hospital renowned for its medical innovation, and its innovation arm, ARC Innovation, have announced a collaborative research agreement with Mana.bio.
- The partnership aims to leverage Mana.bio's AI-powered programmable RNA delivery platform to overcome technological hurdles in RNA therapeutics, paving the way for enhanced treatments for cancer and autoimmune diseases.
Bridging Technological Gaps in RNA
Read More
Life Sciences | News, Analysis, Insights - HIT Consultant
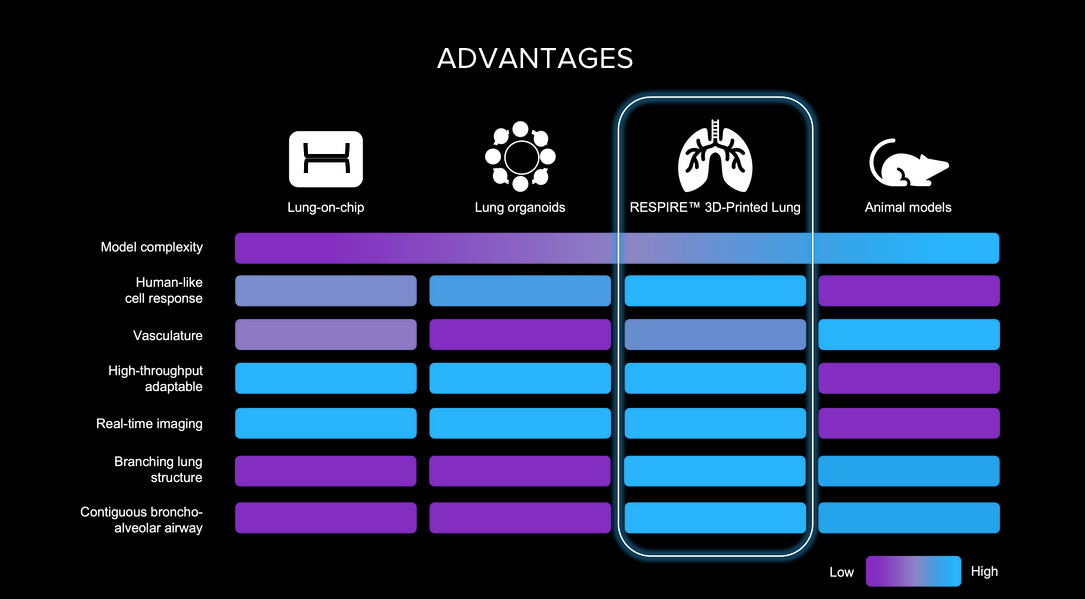
Frontier Bio’s Lab-Grown Lungs Offers Hope for Drug Development
What You Should Know:
- Frontier Bio Corporation has successfully created complex microscale lung tissue by combining 3D bioprinting with the self-assembling capabilities of stem cells, mirroring the natural organ development process.
- Frontier Bio's breakthrough in lab-grown lung tissue signifies a potential turning point in the fight against respiratory diseases and the quest for viable organ transplantation options.
Addressing the Limitations of Animal Testing
Animal
Read More
Medidata Launches Patient Payments: Streamlining Clinical Trial Reimbursements
What You Should Know:
- Medidata, a Dassault Systèmes brand and leading provider of clinical trial solutions to the life sciences industry, today announced the launch of Medidata Patient Payments, a new solution that streamlines trial-related stipends and reimbursements for patients participating in clinical research.
- This offering automates the payment lifecycle and addresses the longstanding challenge of compensating participants for their time, effort, and study-related expenses,
Read More
StudyTeam for Sites Reaches 10,000 Global Research Sites, Streamlining Clinical Trials
What You Should Know:
- OneStudyTeam, a member of the Reify Health portfolio, provides the cloud-based software platform StudyTeam to accelerate the development of new and life-saving therapies. StudyTeam brings research site workflows online and enables sites, sponsors, and other key stakeholders to work together more effectively using common technology.
- The suite of StudyTeam solutions reduces site burden and helps sites pre-screen and enroll more patients, provides sponsors with
Read More
The Role of Stem Cell Therapy in the Future of Personalized Medicine
In the rapidly evolving landscape of healthcare, personalized medicine is making waves by offering tailored therapies that cater to the unique needs of individual patients. One of the most promising advancements in this field is stem cell therapy, particularly autologous stem cell therapy, which utilizes a patient's own cells to promote healing naturally. Unlike traditional treatments, stem cells have a unique ability to enhance the body’s natural healing process, offering a new dimension of
Read More
Medical Experts Utilize NVIDIA-Powered Federated Learning to Advance AI in Tumor Segmentation
What You Should Know:
- A team of experts from leading U.S. medical centers and research institutions is utilizing NVIDIA-powered federated learning to investigate the impact of this technique and AI-assisted annotation in training AI models for tumor segmentation.
- NVIDIA-powered federated learning aims to overcome data-sharing challenges and accelerate the development of more accurate and generalizable AI models in medical imaging.
Federated Learning: Revolutionizing Data
Read More
Verily Expands Wastewater Surveillance Testing to Europe
What You Should Know:
- Verily, a precision health technology company, announced a significant expansion of its wastewater surveillance program into the United Kingdom through an exclusive partnership with Bangor University.
- This collaboration will leverage the university’s wastewater-based public health surveillance program capabilities and main testing lab. Bangor University’s wastewater monitoring capabilities are market leading in the U.K. for environmental bio-surveillance and the
Read More
GI Partners Makes Major Investment in eClinical Solutions
What You Should Know:
- eClinical Solutions LLC, a provider of digital clinical software and services, announced that it has received a majority investment from GI Partners, a private investment firm.
- This investment highlights eClinical Solutions' strong position as a trusted partner for life science organizations of all sizes seeking to accelerate research and advance therapeutic breakthroughs in an increasingly complex trial landscape.
GI Partners' Commitment to
Read More
Sheba Medical, Paradigm Health Partner to Optimize Clinical Trials
What You Should Know:
- Sheba Medical Center, Israel’s largest hospital, and ARC Innovation, the innovation arm of Sheba, has announced a strategic partnership with Paradigm Health to establish a global network for clinical research.
- This collaboration aims to revolutionize the way clinical trials are conducted, making them more accessible, efficient, and patient-centered.
Leveraging AI to Optimize Clinical Trials
The partnership will utilize Paradigm's AI-driven
Read More
Gilead Sciences and Genesis Therapeutics Partner on AI-Driven Drug Discovery
What You Should Know:
- Gilead Sciences, a global biopharmaceutical company, and Genesis Therapeutics, an AI-focused biotechnology firm, have announced a strategic collaboration to discover and develop innovative small molecule therapies.
- The startegic partnership leverages Genesis' cutting-edge AI platform, GEMS, to accelerate drug discovery and development.
A Powerful Collaboration
Genesis Therapeutics' GEMS platform utilizes advanced AI techniques to generate and
Read More