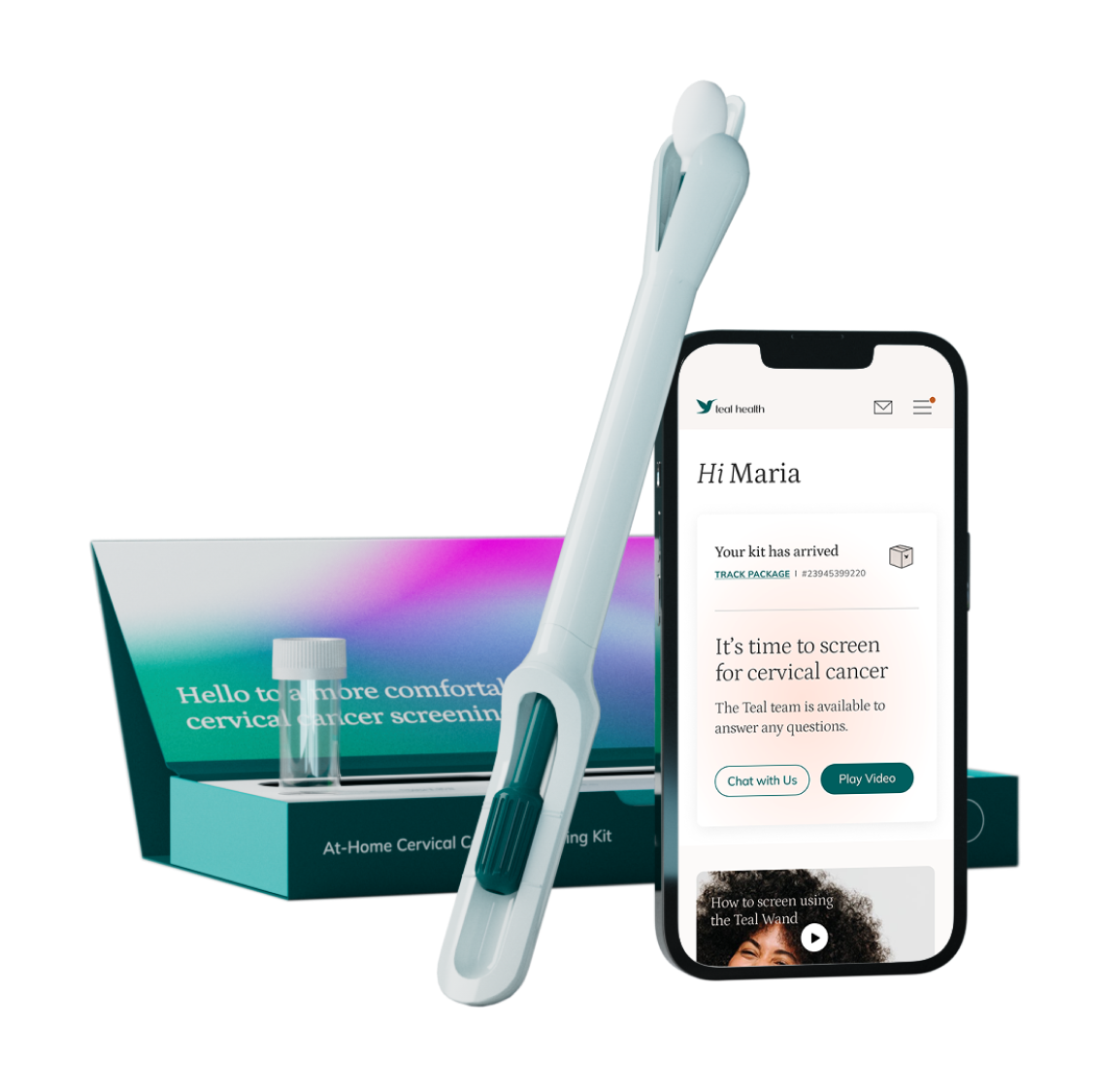
What You Should Know:
– Teal Health®, a women’s health company dedicated to eliminating cervical cancer, today announced a landmark achievement: the U.S. Food and Drug Administration (FDA) has approved the Teal Wand™, marking it as the first and only at-home self-collection device for cervical cancer screening in the United States.
– The FDA approval paves the way for women to have a new, convenient, and effective option for what is commonly known as the Pap smear, directly from the privacy of their homes.
– The Teal Wand™ has demonstrated impressive efficacy, proven to detect cervical precancer 96% of the time, a rate equivalent to clinician-collected samples using a speculum. Designed for individuals aged 25-65 at average risk, the Teal Wand™ will soon be available for direct order via getteal.com. The service includes not just the collection kit but also an end-to-end telehealth support system, providing virtual access to Teal medical providers throughout the screening experience.
Addressing a Critical Gap in Women’s Preventative Health
Cervical cancer is unique in that it is almost entirely preventable with regular screening. Yet, a concerning statistic reveals that more than one in four women in the U.S. are not up-to-date with their recommended screenings. Barriers such as difficulty obtaining time off work, challenges in securing timely appointments, or the discomfort associated with in-clinic pelvic exams often contribute to this gap. The Teal Wand™ aims to overcome these hurdles by offering a comfortable, convenient, and scientifically validated alternative.
“The Teal Wand is a preferred alternative, one that has been built with empathy, is driven by science, and designed to make screening easy, so that more women can take control of their health on their own terms,” the company stated.
How At-Home HPV Screening with the Teal Wand™ Works
Instead of the traditional Pap smear, which has a sensitivity (a measure of accuracy) of around 55%, women using the Teal Wand™ will be testing for the human papillomavirus (HPV). HPV is the virus responsible for nearly all cervical cancers, and Primary HPV testing, with a sensitivity of 95%, is the screening method recommended by current medical guidelines and used by providers in clinical settings. The Teal Wand™ simply offers a different, FDA-approved method for collecting the sample. This approval means women can now use the same highly accurate test as in a doctor’s office, but with the convenience and privacy of at-home self-collection.
Rigorous Science: Validation Through the SELF-CERV Study
Teal Health’s FDA approval was strongly supported by its SELF-CERV study, the largest U.S.-based comparative study of its kind. This pivotal research confirmed that samples self-collected using the Teal Wand™ demonstrate the same performance as samples collected by clinicians in a medical setting.
Crucially, the SELF-CERV study was designed to reflect the racial, ethnic, and socioeconomic diversity of the U.S. population, underscoring Teal Health’s commitment to clinical excellence, equity, and inclusivity in women’s health research. The study also highlighted strong patient preference for the at-home option: 86% of participants stated they would be more likely to stay up to date with cervical cancer screening if they could do it at home, and 94% said they would prefer to self-collect at home with the Teal Wand™ if they knew it was accurate.
“As a mom and a woman, I get how easy it is to put your own health last,” said Kara Egan, CEO and Co-Founder of Teal Health. “That’s why this FDA approval means so much; it’s not just about an innovative new product, it’s about finally giving women an option that actually makes sense for their lives – something that can be done quickly and comfortably at home. Because when we make care easier to get, we help women stay healthy, for themselves and for the people who rely on them every day.”
Availability and Access: Bringing the Teal Wand™ to Women Nationwide
With FDA approval secured, Teal Health is moving swiftly to make the Teal Wand™ accessible. Kits are scheduled to begin shipping in June, initially launching in California and then expanding nationwide as quickly as possible. Teal Health is also actively working with major insurance providers and plans to offer flexible payment options to help alleviate financial barriers and ensure broader access to this innovative at-home screening method.
Women interested in being among the first to know when Teal Health expands to their state can join the waitlist at getteal.com for early access to updates and availability.
