What You Should Know:
– Medidata, a Dassault Systèmes brand and the leading provider of clinical trial solutions to the life sciences industry, today announced a partnership with neuroscience solutions leader Cogstate to reshape clinical trials and outcomes measurement for central nervous system (CNS) diseases across neurodegenerative, psychiatric, motor, and rare neurodevelopmental disorders, among others.
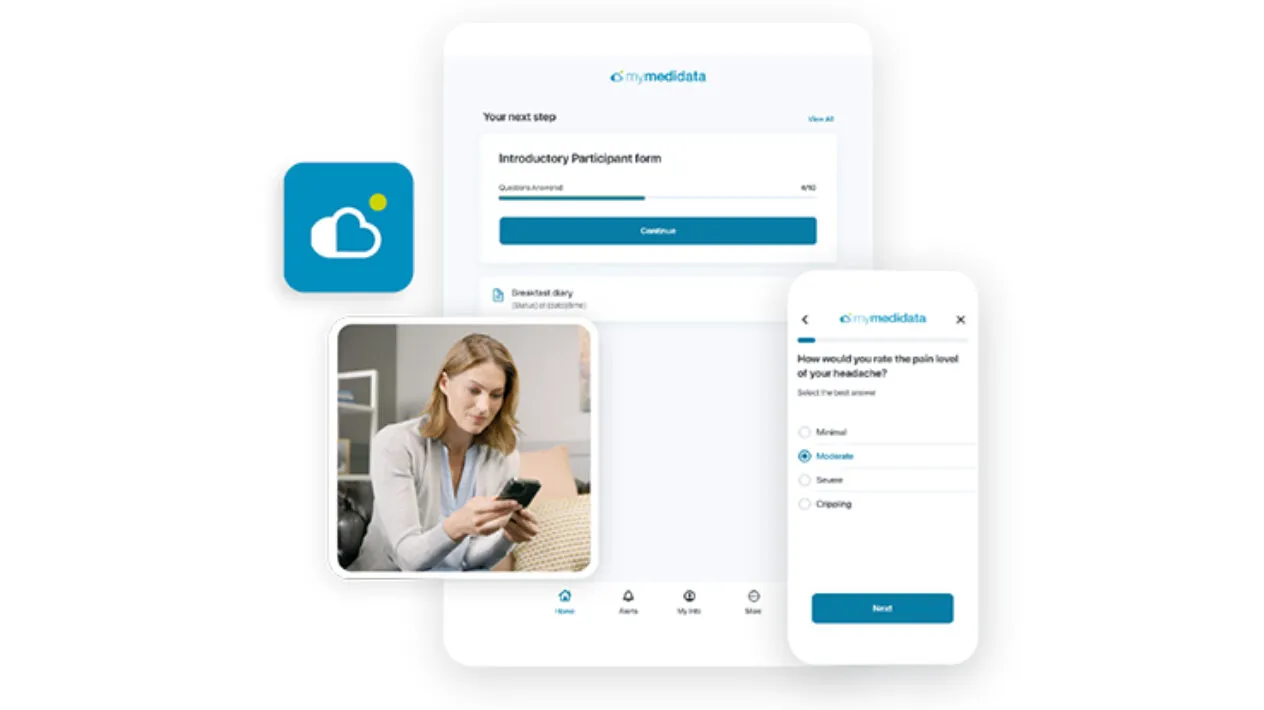
– Together, the companies will deliver an improved experience, supported by Medidata eCOA (electronic clinical outcome assessment) that empowers customers through faster trial starts and optimal rater experiences. This includes a streamlined rater journey through a single mobile device app and a comprehensive suite of data quality assurance solutions for central nervous system (CNS) assessments.
Medidata and Cogstate Partner to Enhance CNS Clinical Trials with a Unified Digital Platform
Medidata, a pioneer in digital solutions for clinical trials, is marking its 25th year of technological innovation, having supported over 34,000 trials and 10 million patients. Trusted by more than 1 million users across 2,200 organizations, Medidata’s comprehensive platform harnesses analytics-powered insights and the world’s largest patient-level historical clinical trial dataset. This collaboration with Cogstate addresses the complexities of CNS (Central Nervous System) clinical trials, delivering a fully integrated platform to enhance assessment delivery and oversight.
The partnership integrates the Medidata Platform with Cogstate’s validated digital cognitive assessments, rater training, and monitoring solutions. The result is streamlined CNS data collection, ensuring increased precision, efficiency, and data quality. Biopharmaceutical companies and clinical trial raters can benefit from this unification by accessing ClinRO, ePRO, and PerfO assessments—including Cogstate’s suite of validated digital tools—through a single device, which simplifies patient outcome monitoring.
The Medidata App, designed specifically for CNS trials, combines Medidata’s Rave EDC and eCOA capabilities with features to optimize CNS study management. Raters using the app can:
– Access enhanced data capture tools, including text entry, handwriting and image capture, and embedded audio recording with smart transcription for increased scoring accuracy.
– Efficiently initiate patient site visits, manage study activities, and perform detailed CNS assessments directly on site tablets.
– Submit CNS assessment data directly into Medidata Rave EDC, which connects seamlessly with Cogstate’s rater monitoring platform, enabling timely review, rater query management, faster trial initiation, and improved data quality monitoring.
Additionally, Medidata’s CNS Suite, which incorporates advanced sensor technology and AI-driven analytics, enables researchers to capture biometric and behavioral data. Through Medidata Sensor Cloud, AI, and the myMedidata platform, this suite offers a patient-centered approach to clinical trials, supporting deeper insights into patient outcomes through a single, life-centered portal.
Leaders from Medidata and Cogstate will share further insights on this collaboration and new CNS trial advancements in a keynote and panel session at Medidata NEXT New York. Together, Medidata and Cogstate continue to innovate in CNS technology, enhancing data capture capabilities and refining patient assessment tools to advance the field of clinical research.
“The subjective nature of evaluating patients and the potential for data variability pose significant challenges in CNS clinical trials,” said Anthony Costello, CEO, Medidata. “Through our joint efforts, and drawing on Medidata’s deep expertise in eCOA, AI, and sensors, we are enabling the highest endpoint data quality standard, simplifying CNS trial setup, and running automated checks on patient assessment transcripts to minimize study team burden and mitigate the potential for errors.”
