What You Need to Know:
– Inato unveils central, searchable repository for all COVID-19 clinical trials designed to support clinical researchers, physicians, and biopharma organizations.
– By centralizing all clinical studies being conducted worldwide and updating trial trends with new information from public registries and aggregators daily, Inato seeks to optimize COVID-19 clinical trial resource allocation and help address any unmet needs as quickly as possible.
Paris-based (and Obvious Ventures-backed) Inato unveiled its anti-covid platform today, a comprehensive, easily searchable, central repository for all existing clinical trials for SARS-CoV2 (the virus responsible for COVID-19). The anticovid platform is public, free to access and offers extensive search and filtering capabilities — a unique and critical feature given the unprecedented pace at which COVID-19 clinical trial research is evolving.
anticovid Platform Background/Methodology
Trials listed in Anticovid mainly come from public
registries or aggregators, including WHO clinical trials registry platform, clinicaltrials.gov, chictr.org.cn, and clinicaltrialsregister.eu.
However, there was to our knowledge no unique and centralized repository
where all clinical studies conducted worldwide on COVID-19 could be
found.
The purpose of Inato’s anticovid
platform is to provide the global healthcare community with easy and
efficient access to any available COVID-19 trial information and research
trends. By centralizing all clinical studies being conducted worldwide and
updating trial trends with new information from public registries and
aggregators daily, Inato seeks to optimize COVID-19 clinical trial resource
allocation and help address any unmet needs as quickly as possible.
Clinical Research for Covid-19
In addition to its detailed search and filtering capabilities, the anticovid platform analyzes the latest COVID-19 clinical trial information, providing platform subscribers new analysis twice per week. Most recently, the platform deduced that:
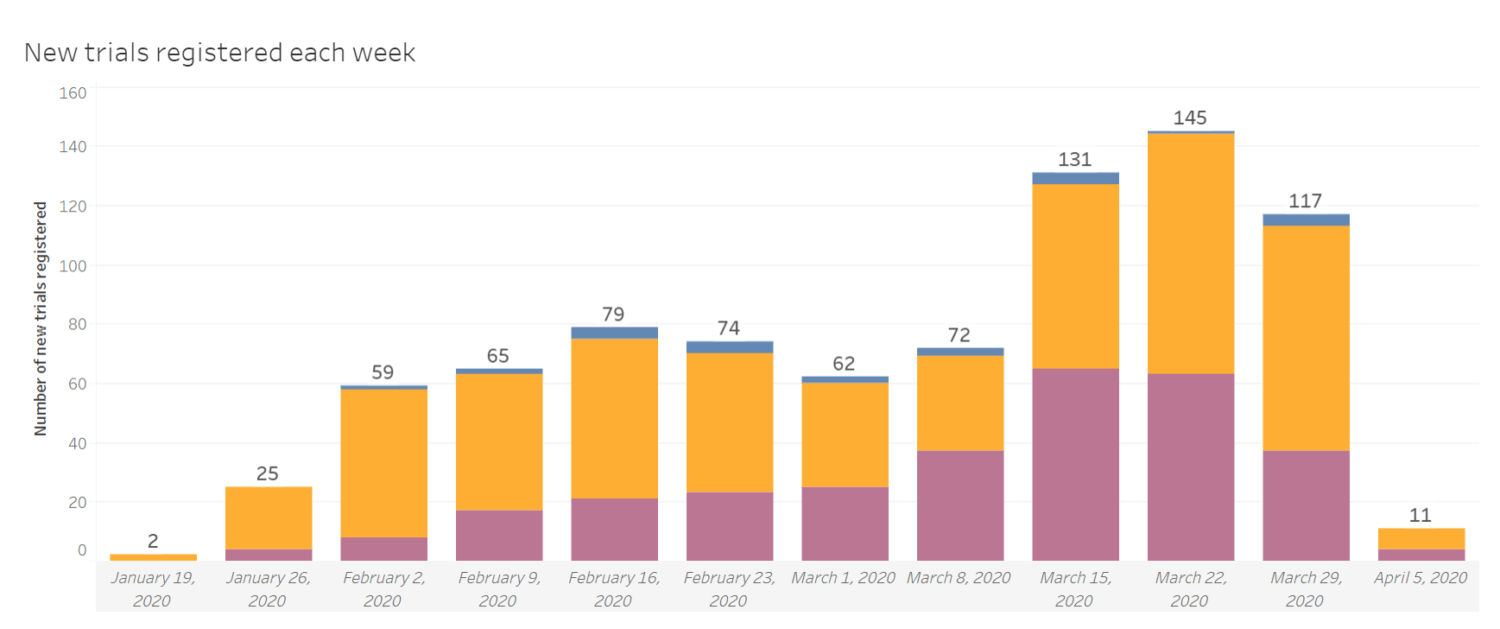
– Nearly 1,000 COVID-19 trials were launched or anticipated in the past 4 months. This equates to an average of 210 trials per month, compared to just 40 breast cancer trials per month and 1 malaria trial per month.
– While China remains responsible for the most trials, other highly affected, developed countries are quickly gaining speed. For example, Italy, France and the U.S. have significantly grown their contributions in recent weeks.
– Early signals suggest some antiviral agents could be ineffective for treating COVID-19 (at least in seriously ill patients), however a significant focus of trials remains on those drugs.
– Chloroquine/hydroxychloroquine is also a focus, with more than 50,000 participants currently participating in this therapeutic class of trials in the U.S.
– The most frequently tested therapeutic classes are antiviral agents, Chloroquine/Hydroxychloroquine and traditional/Chinese medicine
“We’ve been tracking COVID-19 clinical trials since January and noticed how difficult it was to consolidate, compare and take action on the hundreds upon hundreds of trials being developed,” said Inato Co-Founder and CEO Kourosh Davarpanah. “Our company’s objective is to bring new doctors and their patients into the clinical research ecosystem to unlock the potential of unengaged research sites so biopharma can bring innovative therapies to market faster. With our anticovid platform, we’re taking things one step further by centralizing all COVID-19 clinical trial information and making it easy to navigate so that clinical researchers, physicians and biopharma companies can more efficiently participate in the fight for a cure.”
Availability
To access Inato’s anticovid platform, visit https://covid.inato.com/analysis.
