
What You Should Know:
– Parasym has launched Nuropod, the first ear-based, clinically studied vagus nerve stimulation (VNS) device in the U.S. that does not require surgery.
– The wearable device is designed to reduce symptoms such as persistent fatigue, anxious states, and markers related to inflammation by activating the vagus nerve to restore the body’s “rest and digest” state. The device is backed by more than 50 completed clinical studies.
A Non-Surgical Alternative to Vagus Nerve Stimulation
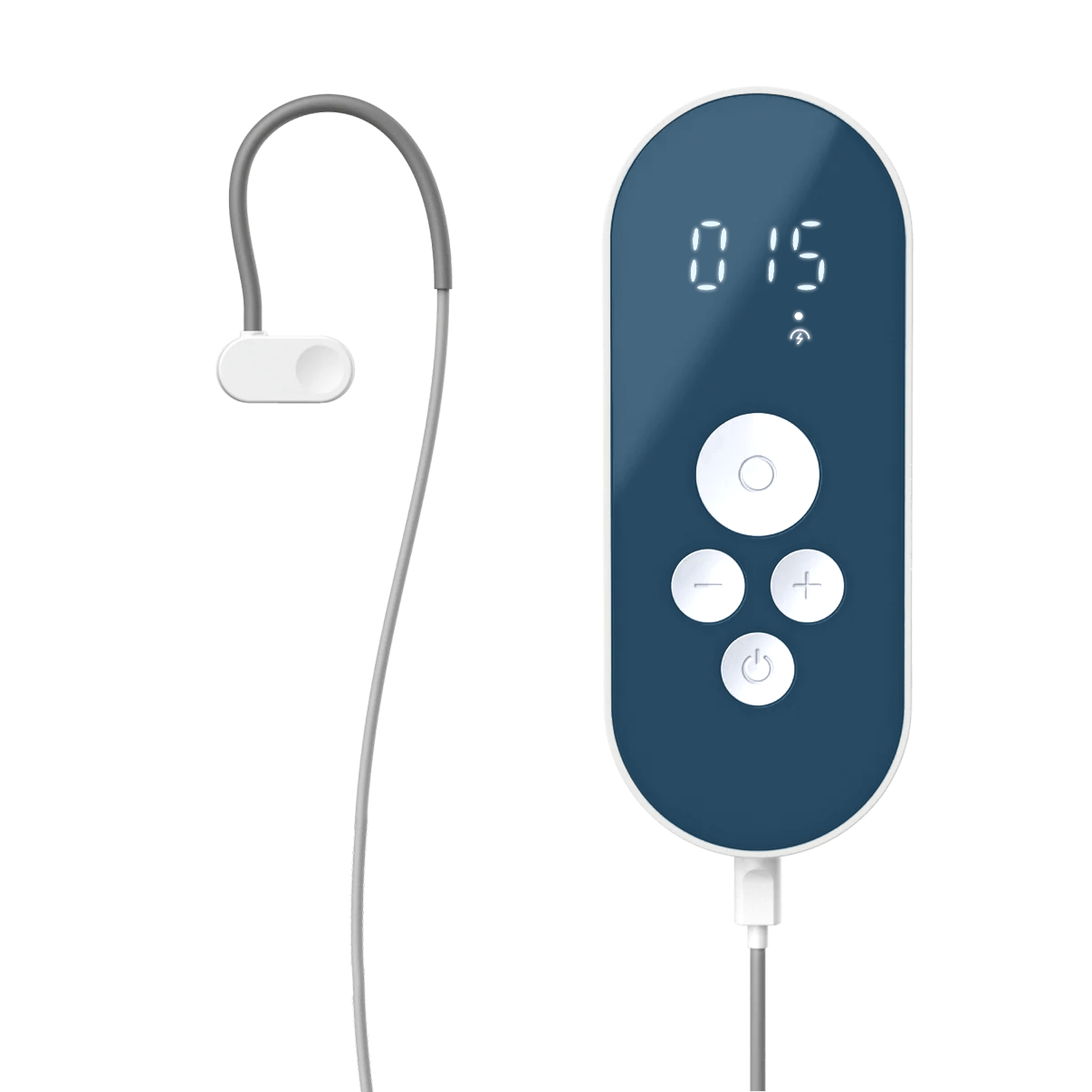
Vagus nerve stimulation has been shown to regulate the autonomic nervous system, which is the body’s command center for stress, recovery, and inflammation. Historically, accessing clinically proven VNS required a costly surgical implant. Nuropod, however, delivers Auricular Vagal Neuromodulation Technology (AVNT), a proprietary method that targets the vagus nerve through the ear. This method has been shown in peer-reviewed studies to be as effective as, or even more effective than, the surgical approach.
According to Dr. Elisabetta Burchi, Parasym’s Head of Research, Nuropod was designed to help people reset their nervous systems. She noted that nearly one in three Americans reports feeling constantly fatigued, and the average adult in the U.S. has more than 40 hours of sleep debt each month.
Clinically Proven Results and FDA Designation
AVNT has shown significant results in multiple randomized, placebo-controlled studies. These results include:
- A 61% increase in vagus nerve activity in 5 minutes.
- A 78% decrease in inflammation in the body.
- A 48% decrease in fatigue.
- A 19% increase in sleep quality.
- A 35% decrease in anxious thoughts.
- A 32% improvement in memory.
Collaborations with institutions like Harvard and UCLA have studied AVNT for reducing inflammation, improving cardiovascular function, enhancing cognitive performance, and alleviating fatigue in conditions such as long COVID and POTS. The device has also been granted an FDA Non-Significant Risk Designation, which affirms its safety.
Availability/Pricing
Nuropod offers a science-backed, easy-to-use device that fits into a user’s daily life, from professional athletes tracking their recovery to people seeking relief from persistent symptoms. The device is available direct-to-consumer for $900, offering the same clinically proven approach that was previously only available through surgical implants.
