What You Should Know:
– Reprieve Cardiovascular, a clinical-stage company secures $61M in Series B funding led by Deerfield Management and included participation from several other investors, such as Arboretum Ventures, Lightstone Ventures, and an undisclosed strategic investor.
– The funding round, which combines equity investment with a debt facility will be used to support the rapid execution of the global FASTR II pivotal clinical trial and to fund key commercial readiness activities.
Intelligent Decongestion Management
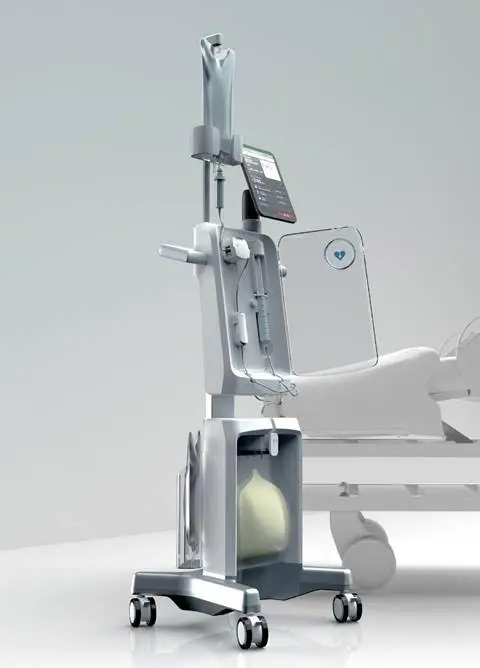
Reprieve Cardiovascular is pioneering an intelligent decongestion management therapy for Acute Decompensated Heart Failure (ADHF). ADHF is a serious condition characterized by symptoms like difficulty breathing, fatigue, and swelling, often leading to unplanned hospitalizations. Managing fluid removal is a persistent challenge, as the primary treatment of diuretics is hard to administer precisely, which can lead to kidney injury, longer hospital stays, and frequent readmissions.
The company’s proprietary Reprieve system is designed to personalize decongestion management by safely, quickly, and thoroughly removing excess fluid. It works by administering diuretics with precision while replenishing the body with saline to support optimal kidney function. The system uniquely combines real-time physiological monitoring with automated recommendations, allowing physicians to tailor treatment to each patient’s specific needs.
FASTR II Pivotal Clinical Trial
The new funding will directly support the FASTR II pivotal study, which has now enrolled its first patient. The trial will evaluate the Reprieve system’s efficacy against optimal diuretic therapy in patients hospitalized with ADHF. The main goal is to determine if the Reprieve system can decongest patients more effectively than the current standard of care. The study plans to enroll up to 400 patients across the United States and Europe and will support a future premarket approval submission in the U.S..
Mark Pacyna, CEO of Reprieve Cardiovascular, said the capital raised ensures the company is “positioned to generate the clinical and economic evidence essential for regulatory approval and commercialization”. He believes their personalized approach can lead to “better outcomes for both patients and healthcare systems around the world”.
