What You Should Know:
– The Centers for Medicare & Medicaid Services (CMS) launches its Cell and Gene Therapy (CGT) Access Model, a new approach to delivering cutting-edge treatments for people on Medicaid living with sickle cell disease.
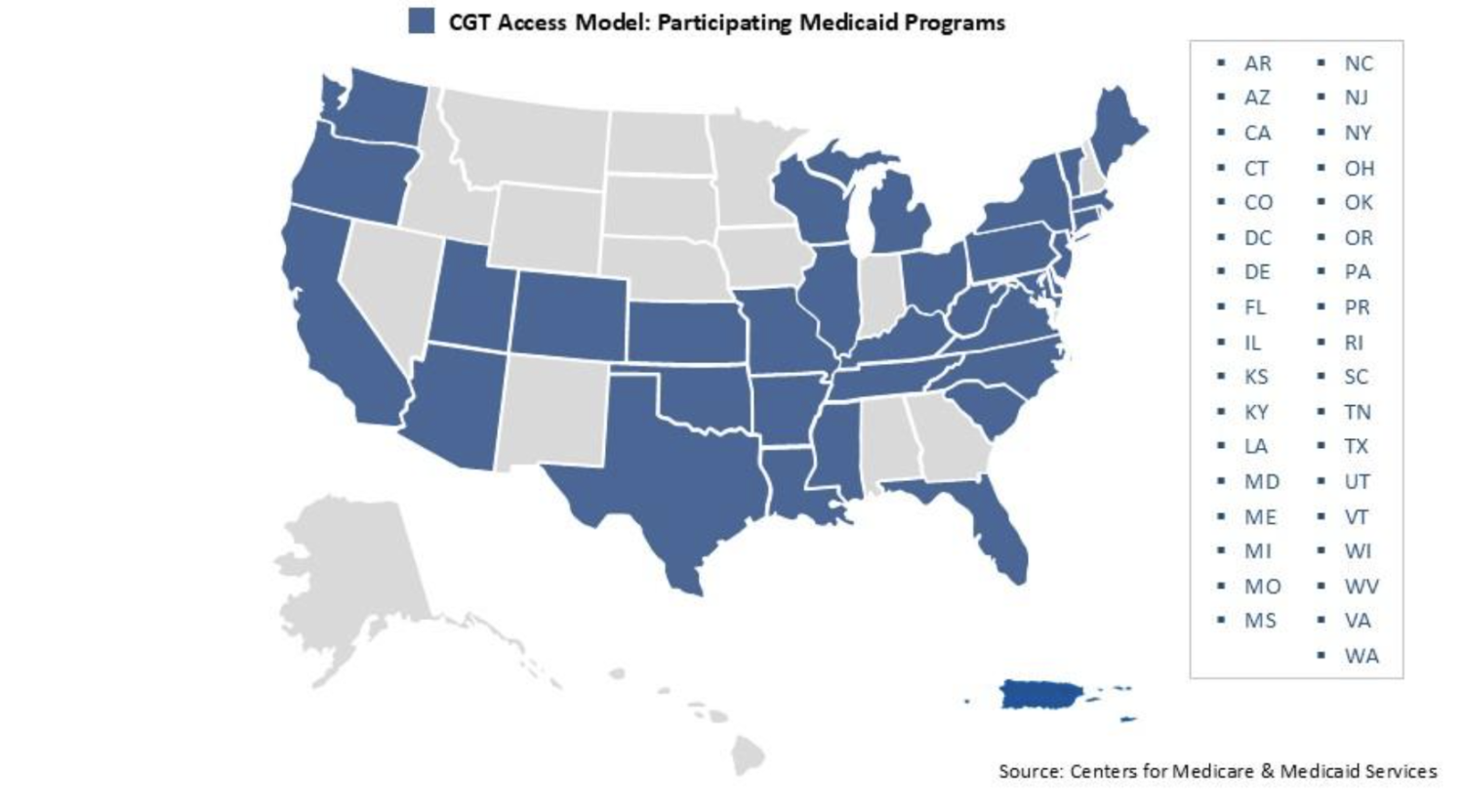
– A total of 33 states, plus the District of Columbia and Puerto Rico, will participate in this model, collectively representing approximately 84% of Medicaid beneficiaries with the condition, significantly expanding access to transformative care.
Outcomes-Based Agreements for Innovative Therapies
Led by the CMS Innovation Center, the CGT Access Model marks the first time the federal government has negotiated outcomes-based agreements with CGT manufacturers on behalf of state Medicaid agencies. Under this innovative model, participating states will receive guaranteed discounts and rebates from participating CGT manufacturers if the therapies fail to deliver their promised therapeutic benefits.
Sickle cell disease is known as an extremely painful condition that significantly impacts overall quality of life. It can lead to severe long-term health complications, including stroke, acute chest syndrome, and chronic end-organ damage, which often result in higher rates of emergency department visits, hospitalizations, and increased long-term healthcare expenditures.
Key Features and Participating States
The CGT Access Model includes several key features designed to facilitate its success:
- CMS-negotiated outcomes-based contracts with manufacturers, developed with input from state Medicaid agencies, patients, and providers.
- Optional federal support of up to $9.55 million per state to aid with implementation, outreach, and data tracking.
- Flexible start dates for participating states, ranging between January 2025 and January 2026.
- Potential future expansion to cover other diseases that rely on high-cost, high-impact therapies.
Participation in the model is voluntary for both states and manufacturers. Manufacturers who responded to CMS’ Request for Applications and agreed to negotiate terms based on clinical outcomes were eligible to participate. This initiative underscores CMS’ commitment to accelerating access to innovative therapies, improving patient health, and ensuring the smart use of Medicaid resources.
The states and territories participating in the CGT Access Model are: Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Illinois, Kansas, Kentucky, Louisiana, Maine, Maryland, Michigan, Mississippi, Missouri, New Jersey, New York, North Carolina, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, and Wisconsin, as well as the District of Columbia and Puerto Rico.
“This agreement is a major win for American patients and for Medicaid to provide patients new access to groundbreaking therapies for sickle cell disease,” said U.S. Health and Human Services Secretary Robert F. Kennedy, Jr. “Thanks to the leadership of Dr. Oz, CMS is making this model a reality. I look forward to seeing states lead the charge to improve health outcomes at lower costs for the American people.”
