What You Should Know:
– Pelvital USA, Inc., a leading women’s health innovator, has announced the successful closing of an oversubscribed $5M seed-plus funding round. The investment round was led by Boomerang Ventures with new investors including Pier 70 Ventures, Life Science Angels, Tech Coast Angels Orange County, and Blue Pacific Fund.
– This additional $2.32M funding will significantly accelerate the commercialization of Flyte, their novel treatment for stress urinary incontinence (SUI).
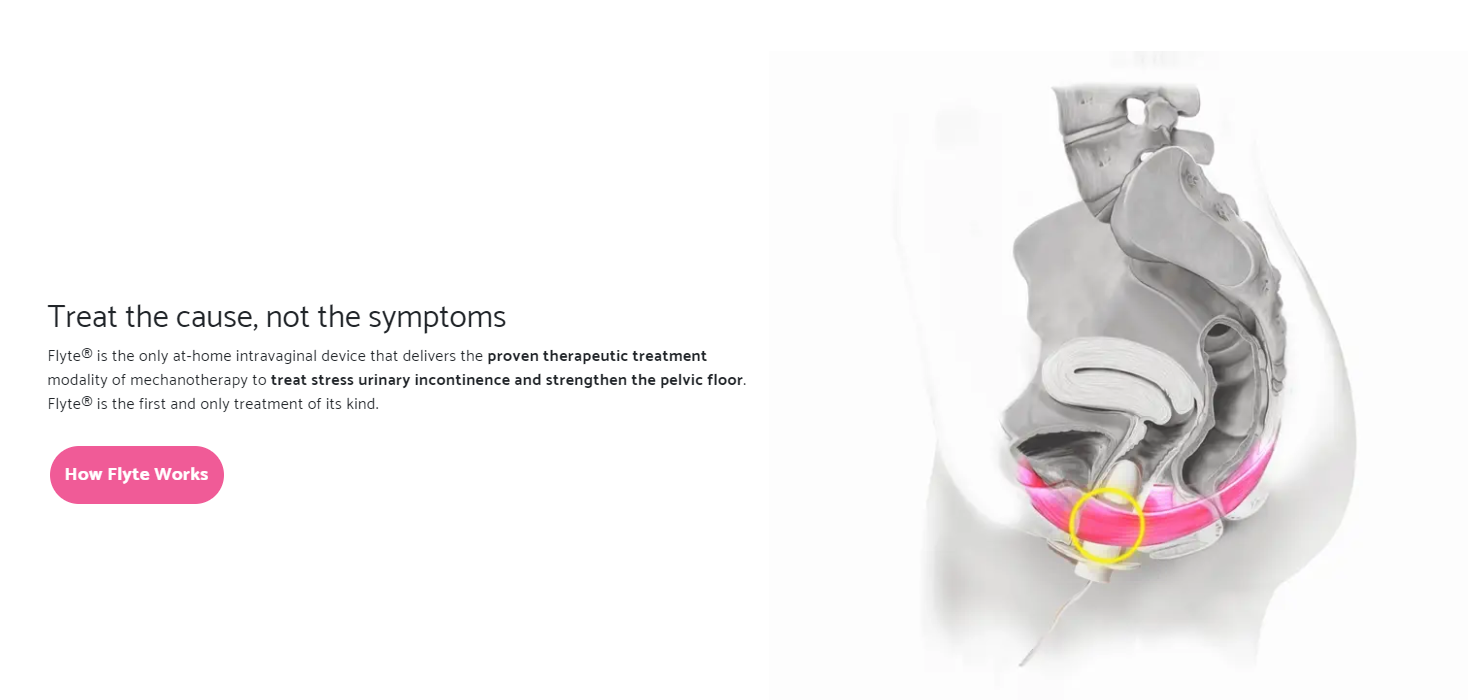
Flyte: A Game-Changer for Women with SUI
Flyte is a revolutionary, FDA-cleared, and clinically proven treatment designed to address SUI and strengthen pelvic floor muscles. This accessible and easy-to-use solution offers women a non-invasive option to manage this common condition. The typical treatment regimen involves using the device for only five minutes a day, for six weeks. While FDA-cleared for over-the-counter use, Flyte is often prescribed by clinicians, ensuring optimal patient care and guidance.
Clinically Validated Efficacy and Durability
Two published clinical trials have demonstrably validated Flyte’s safety, effectiveness, and long-lasting treatment benefits for women with SUI. A recent study published in Therapeutic Advances in Urology revealed that 71% of participants achieved dry or near dry status after using Flyte for just 2-12 weeks, as evidenced by a reduction in 24-hour pad weight.
“Completing this round is an important step in continuing Pelvital’s unwavering dedication to provide women with innovative solutions for pelvic health, including the treatment of SUI,” says Lydia Zeller, President and CEO of Pelvital. “This funding will play a crucial role in accelerating our commercialization of Flyte with a strong emphasis on expanding payor coverage and enhancing clinical education and clinician awareness.”
