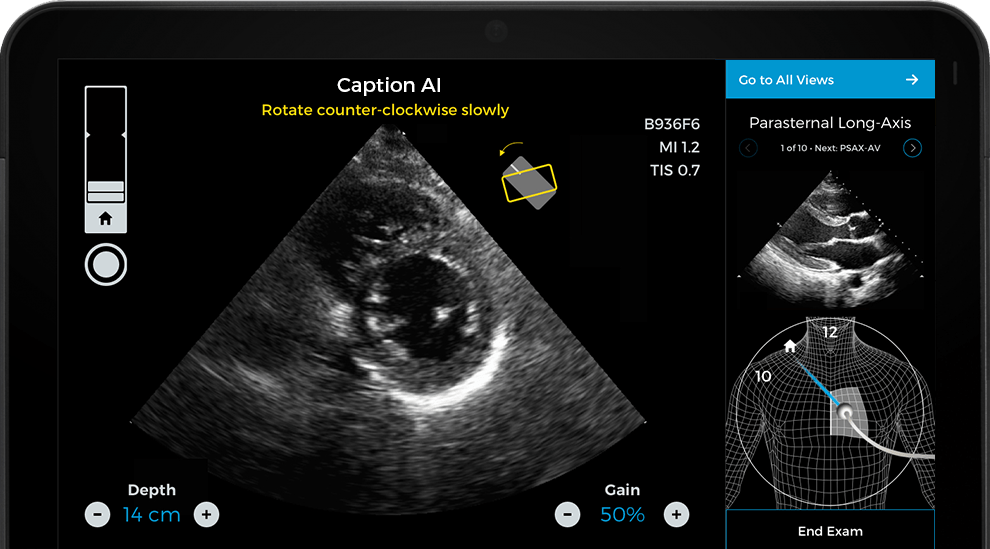
What You Should Know:
– Caption Health, the leader in using artificial intelligence (AI) and services to improve heart ultrasound access, today announced that it has received a CE Mark for its Caption AI™ technology platform.
– This certification represents the first step in making Caption Health’s industry-leading technology platform available outside the US, and highlights the company’s strong clinical and regulatory track record as it uses AI and ultrasound to deliver better cardiac care.
AI-Driven Ultrasound Program
Caption Health is making earlier cardiac care accessible wherever patients are, utilizing the Caption AI platform to provide a convenient, cost-effective solution for cardiac ultrasound, the primary tool for assessing heart failure. But before the platform became widely available, patients could only be diagnosed after trained sonographers performed an ultrasound in a hospital or specialized setting – typically after the onset of symptoms. Today, it can be used at scale to detect signs of heart failure in at-risk patients in doctors’ offices and even at home, helping to prevent avoidable hospitalizations and support improved clinical outcomes.
Caption AI enables medical staff to obtain quick, easy, and accurate assessments of cardiac function and ejection fraction wherever patients are, and has been widely available in the U.S. since it received FDA 510(k) clearance in 2020.
“Our technology harnesses the power of ultrasound and allows diagnostic images to be acquired wherever the patient may be. Earlier detection of cardiac issues allows us to impact the course of disease and improve outcomes,” said Randolph P. Martin, FACC, FASE, FESC, Chief Medical Officer of Caption Health. “This is a big step forward in improving access and addressing the significant unmet need stemming from the increasing global burden of cardiovascular diseases like heart failure and valvular heart disease.”
Milestones
Medical devices and software require the CE Mark for clinical applications in several key regions including the UK and EU. “Our platform is one of the first AI-based ultrasound products to obtain a CE Mark through the robust and comprehensive European Medical Device Regulation (EU MDR) process. Our strong clinical foundation facilitated a seamless pathway towards certification through the EU MDR clinical evaluation requirements,” commented Tahir Rizvi, Head of Regulatory Affairs and Quality Assurance at Caption Health. “This milestone will help us address the increasing global burden of disease from conditions such as heart failure, valvular heart disease, and coronary artery disease.”
