What You Should Know:
Propeller Health has been awarded FDA 510(k) clearance to connect patients using AstraZeneca’s Symbicort Inhaler to Propeller, enhancing medication coverage and respiratory digital health.
The sensor for Symbicort, a controller medication for asthma and COPD, was developed in partnership with AstraZeneca. With this new medication on our roster, Propeller now connects to 90 percent of the inhaled medications for asthma and COPD on the U.S. market.
Propeller Health, a Madison, WI-based digital health company dedicated to the management of asthma and COPD, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to connect patients using the AstraZeneca’s Symbicort Inhaler to its digital health platform.
Doctor-Recommended Way to Manage Your Asthma or COPD
Normally, the airways of your lungs are open, allowing the air you breathe to move in and out easily. When you have asthma, exposure to inhaled irritants, or triggers, can cause the walls of your airways to become inflamed and the muscles around the airways to tighten up. This makes your airways narrower, leaving less room for air to flow.
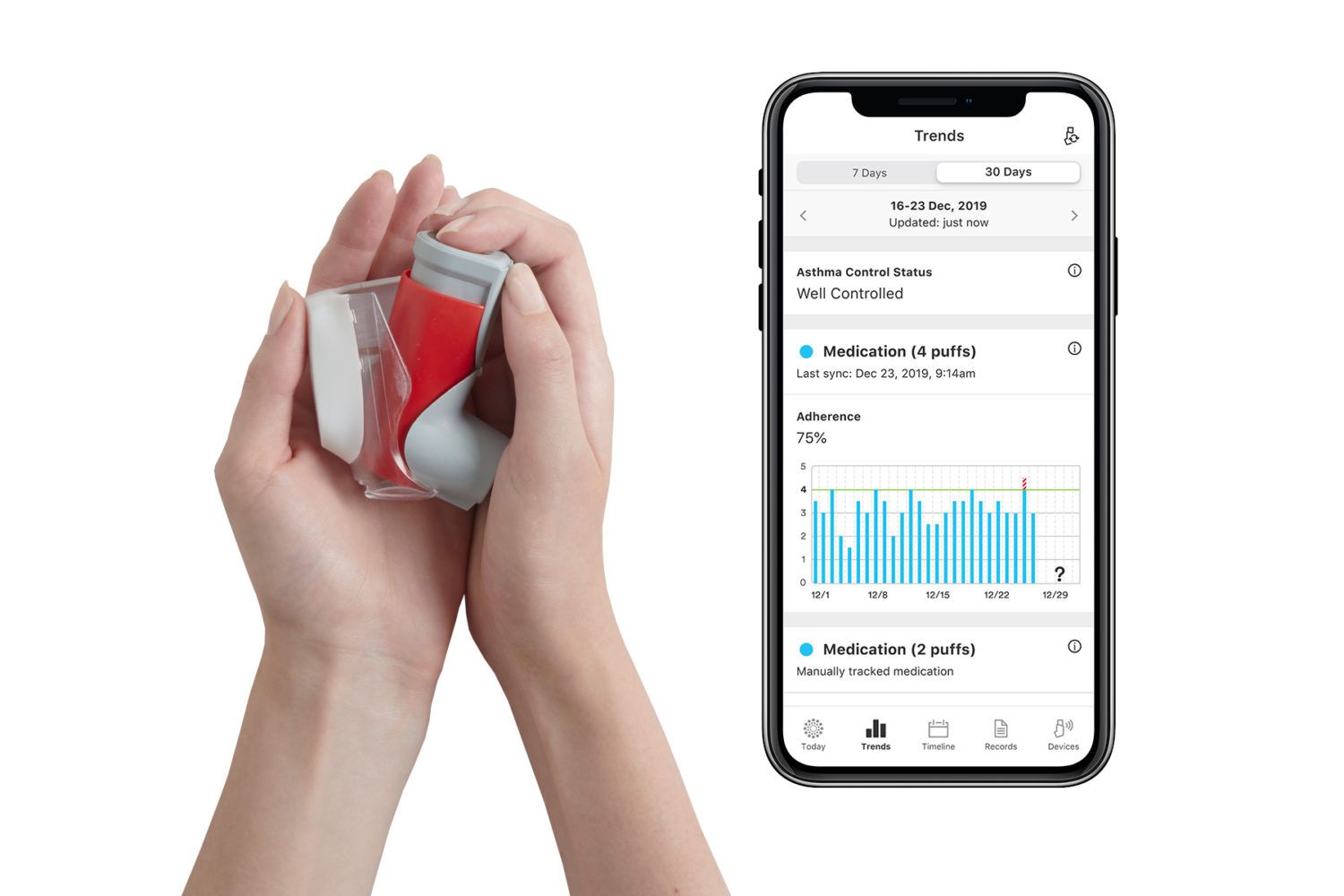
Propeller is a digital health tool that helps people with asthma or COPD manage their condition in partnership with their clinician. Propeller sensors attach to patients’ existing inhalers and deliver insights on medication use to the Propeller app on their smartphone, which patients can then share with their clinician to help inform their treatment plan.
Today, Propeller’s platform already connects to the majority of inhalers used by asthma and COPD patients, including those manufactured by Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Orion, as well as many generic equivalent inhalers.
Clinical Improvements & Outcomes
Reductions in rescue inhaler use translate to better asthma and COPD control, lower healthcare costs, more days free from symptoms, and higher patient quality of life. Propeller’s consistent improvements in disease control and medication adherence are seen across diverse patient populations, settings, and geographies.
The Propeller platform has been shown in previous clinical studies to increase asthma control by up to 63 percent, increase medication adherence by up to 58 percent, reduce asthma-related emergency department visits and hospitalizations by as much as 57 percent, and reduce COPD-related healthcare utilization by as much as 35 percent. To date, Propeller has been studied in 25+ clinical studies totaling over 2,500 patients and has been deployed in more than 40 commercial programs across the US to date.
“Our partnership with AstraZeneca will give respiratory patients a tool to help manage their condition and increase their medication adherence, a critical factor in keeping people out of the hospital,” said David Van Sickle, co-founder and CEO of Propeller Health. “This is an important step in transforming the way people receive preventative care, enabling self-management from home and ensuring that providers have the bandwidth to focus on high-risk patients.”
