
New non-invasive, saliva-based glucose test measures glucose in saliva rather than blood could eliminate finger pricks for diabetes management.
The iQ Group Global, a Sydney, Australia-based consortium of life science and financial services companies has unveiled the Saliva Glucose Biosensor, a non-invasive, saliva-based glucose test for diabetes management that measures glucose in saliva rather than blood. The saliva-based glucose test could eliminate painful finger pricks for diabetes management.
The Problem: Painful Finger Pricks
Diabetes is a metabolic disease defined by high blood sugar levels and can affect organs such as the heart, eyes, and kidneys. According to the World Health Organization, over 420 million people suffer from this condition worldwide. People with diabetes must monitor their blood glucose levels regularly and frequently, but the existing method is invasive, painful, and inconvenient. Patients avoid frequent glucose testing as it requires taking a blood sample from the patient’s fingertip.
The Solution: Saliva Glucose Biosensor
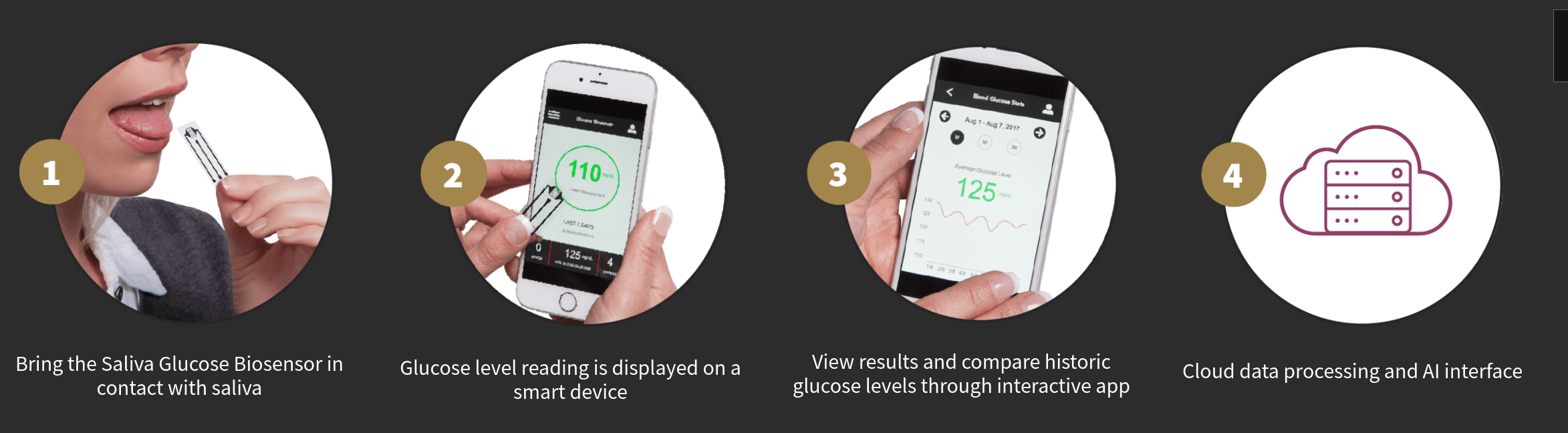
The Saliva Glucose Biosensor comprises the Glucose Biosensor Unit and a mobile health app. The Glucose Biosensor Unit is a small, disposable strip, which when exposed to an individual’s saliva instantly provides a glucose measurement. The glucose measurement will be presented in real-time, via a proprietary digital app on a patient’s smart device. The biosensor exhibits high sensitivity hence is able to detect glucose levels at considerably lower levels than in blood. It has a linear glucose sensing capability at concentrations of 100 times lower than current blood measuring methodologies.
The Saliva Glucose Biosensor was invented by Professor Paul Dastoor and his team at the Centre of Organic Electronics at the University of Newcastle in Australia. The iQ Group Global acquired the biosensor technology in 2016 and has accelerated its development for diagnostic applications.
Pilot Research Program
The iQ Group Global is currently working on a pilot research and development program with the University of Newcastle to expand beyond the saliva glucose diagnostic test and develop the platform of Point of Care Diagnostic Tests beginning with tumor markers, hormones, and communicable diseases.
The solution will open significant opportunities to improve the way diabetes is monitored and managed, enabling patients to store and analyze their data, share monitoring data with their healthcare team or relatives, create and send automated reminders when it is time to test glucose levels, offer educational services, and act as a provider for healthcare companies who offer patient support programs.
“Diabetes is a global epidemic, with 1 in 11 adults living with the disease. Achieving normoglycemia is one of the main targets for diabetes patients. However, finger prick testing is a painful and frustrating process, with many citing the pain as the main reason for poor adherence to testing protocols. By eradicating the need for finger prick blood tests, the saliva-based test will lead to increased glucose monitoring and better healthcare outcomes among these patients,” said Dr. George Syrmalis, Chief Executive Officer and Chairman of The iQ Group Global.
