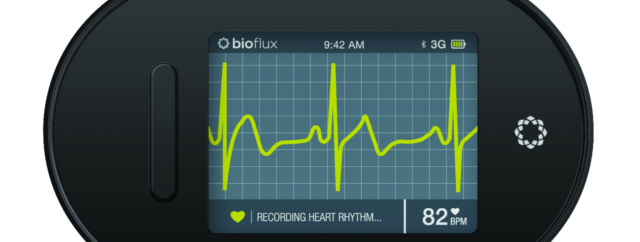
Biotricity today announced the commercial availability of Bioflux, a real-time, high-precision mobile cardiac telemetry solution for customers. The Bioflux system, which received final FDA approval in December, is a complete solution for cardiac monitoring and diagnosis, consisting of the Bioflux device, proprietary software, and a 24/7 monitoring center that merges seamlessly with physicians’ existing platforms and workflows.
The Bioflux system is a complete solution for cardiac monitoring and diagnosis, consisting of the Bioflux device, proprietary software, and a 24/7 monitoring center that merges seamlessly with physicians’ existing platforms and workflows. Unlike traditional cardiac monitoring solutions, Bioflux extends the support a patient receives at a care facility into the patient’s home.
The device monitors a patient’s ECG in near real-time, constantly analyzing and collecting data on the device and periodically uploading to the cloud via embedded cellular technology. Both symptomatic and asymptomatic patient symptoms are reviewed and triaged for each patient throughout the monitoring period. In the past, patient data from cardiac monitoring solutions were not reviewed in near-real time, resulting in potentially life-threatening delays. As a medical device, Bioflux provides immediate, validated clinical data that aids in the diagnosis of cardiac arrhythmias.
“Bioflux was purposely built for cardiac patients who require active monitoring because, until now, short-term monitoring or admission into the hospital for long-term observation was the standard,” said Mr. Waqaas Al-Siddiq, Founder and CEO of Biotricity in a statement. “Our technology addresses the multibillion-dollar cardiac-monitoring market. It does this in a truly innovative way, by actively and securely collecting patient data in real-time and periodically transmitting it for analysis within the cloud. Our technology seamlessly communicates data bi-directionally over a secure communications network to facilitate superior data analysis and integration into healthcare providers’ workflow, which can improve patient-consumer outcomes by providing their clinician with actionable data and feedback.”
