Great Lakes NeuroTech (GLNT) has announced plans to collaborate with the pharmaceutical company UCB to provide individuals with Parkinson’s Disease (PD) and their clinicians with improved quantitative tools for assessing the impact of their current treatment with the aim of providing better individual patient experiences and, ultimately, improving quality of life.
Parkinson’s causes motor symptoms of tremor, slowed movements, and impaired mobility, with side effects of medication including involuntary movements. These symptoms can vary significantly, both in when during the day they occur and in their severity, which can be challenging for individuals managing their condition. The ability to provide detailed feedback about how a patient is responding to treatment could allow clinicians to better tailor care programs and adapt medicine doses to suit individual patient circumstances.
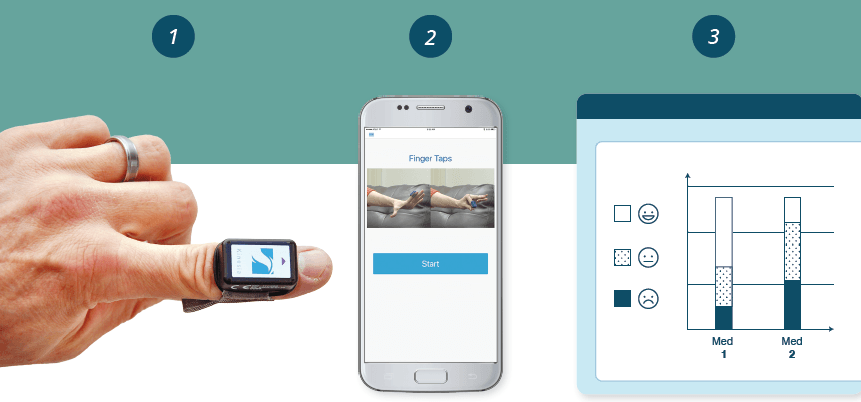
To tackle this challenge, UCB and GLNT will jointly explore ways to combine data from objective wearable diagnostics (sensors and apps) and therapy dosage into visualization feedback tools for clinicians and patients. These visual tools have the potential to help clinicians adjust medication dosage to optimal settings and direct patient feedback to confirm the therapy is working.
The first step in this collaboration is a pilot study, where both partners will bring their respective expertise:
• GLNT’s Kinesia system for objective, wearable assessment of Parkinson’s motor symptoms will provide the technology foundation of sensors and apps for remote symptom monitoring.
• UCB’s NEUPRO® therapy, the first Parkinson’s transdermal solution administered as a patch applied directly on the skin, releases rotigotine, a dopamine agonist medicine, providing continuous stable delivery of the drug over a 24 hour period.
“UCB is committed to identifying and addressing the unmet needs of people living with Parkinson’s Disease to enable them to have a more engaged life every day”, said Ana Infante, Head of UCB’s Free Motion Mission. “We are excited to be collaborating with GLNT to progress and explore value creating opportunities in movement disorders and other neurological diseases of high unmet need. This partnership supports our vision of ensuring all patients with movement disorders experience an optimum treatment experience.”
GLNT’s Kinesia product line provides remote monitoring of Parkinson’s through wireless, wearable sensors and smart phone applications. The technology has been validated with over 100 publications and has FDA and EU clearance for use.
