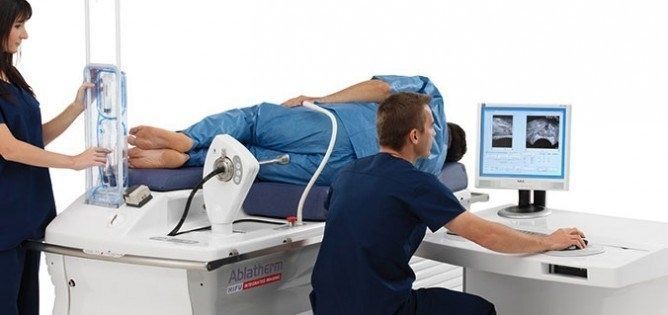
The FDA has cleared the non-surgical, non-invasive treatment for prostate cancer known as HIFU or High Intensity Focused Ultrasound Therapy. The Food and Drug Administration approved HIFU after more than 10 years of clinical trials, paving the way for Ablatherm HIFU to become the treatment of choice for localized prostate cancer in the U.S. More than 40,000 patients worldwide have had HIFU treatments since the procedure was approved in Europe. The procedure is also approved by Health Canada.
The Ablatherm Integrated Imaging device precisely targets the tumor through a computer controlled rectal probe. Ultrasound waves destroy the prostate tissue with no damage to the surrounding organs. This proven treatment option is effective, efficient and adaptable, with early success determination and minimal side effects.
The first North American provider of this technology and treatment, Maple Leaf HIFU, has the largest North American experience in treating prostate cancer patients with Ablatherm HIFU. Patients from the U.S. and Canada are treated at the world class Cleveland Clinic Canada location in downtown Toronto. The lead Urological Surgeon, Dr. William Orovan explains, “Ablatherm HIFU has the best patient safety outcomes by far and cure rates are excellent. Side effects of HIFU, including incontinence and erectile dysfunction, are much less than traditional surgery or radiation.”
The American Cancer Society lists prostate cancer as the number one cancer affecting men in North America. In 2015, 200,000 men will be diagnosed with the disease and over 25,000 will die. Ablatherm-HIFU is a single treatment intended for men in stage T-1 or T-2 of organ confined prostate cancer. HIFU can be repeated if necessary and is used to treat patients who have failed radiation therapy.
